Chemical (Alkali and Acid) Injury of the Conjunctiva and Cornea
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
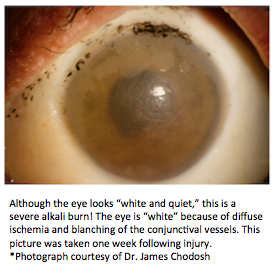
Chemical (alkali and acid) injury of the conjunctiva and cornea is a true ocular emergency and requires immediate intervention. Chemical injuries to the eye can produce extensive damage to the ocular surface and anterior segment, leading to visual impairment and disfigurement. Early recognition and treatment ensure the best possible outcome for this potentially blinding condition.
Disease Entity
International Classification of Diseases
ICD-9-CM: 940.2 alkaline chemical burn to cornea and conjunctiva; 940.3 acid chemical burn to cornea and conjunctiva; 372.06 chemical conjunctivitis. ICD-10-CM: T26.60XA corrosion of cornea and conjunctival sac, unspecified eye, initial encounter.
Epidemiology
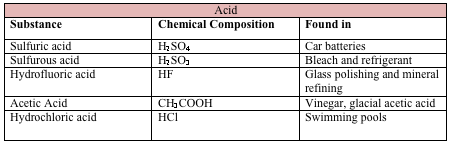
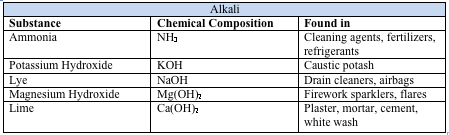
Chemical injuries to the eye represent 11.5%-22.1% of ocular traumas.[1] About two-thirds of these injuries occur in young men, and children ages 1-2 years are particularly at risk. The vast majority of the injuries occur in the workplace as a result of industrial accidents. A minority of injuries occur in the home or secondary to assault. Alkali materials are found more commonly in building materials and cleaning agents, and alkali injuries occur more frequently than acid injuries.[2]
Etiology
Chemical injuries occur as a result of acid, alkali, or neutral agents, with alkalis being responsible for 60% of these injuries. Common causes of alkali and acid injuries are listed below.[2][3]
Pathophysiology
Alkali
Alkali agents are lipophilic and therefore penetrate tissues more rapidly than acids. They saponify the fatty acids of cell membranes, penetrate the corneal stroma, and destroy proteoglycan ground substance and collagen bundles. The damaged tissues then secrete proteolytic enzymes, which lead to further damage.[4][5]
Acid
Acids are generally less harmful than alkali substances. They cause damage by denaturing and precipitating proteins in the tissues they contact. The coagulated proteins act as a barrier to prevent further penetration (unlike alkali injuries).[5] The one exception to this is hydrofluoric acid, where the fluoride ion rapidly penetrates the thickness of the cornea and causes significant anterior segment destruction.[6]
Primary Prevention
Because the majority of injuries occur at work, protective eye shields are mandatory when handling potentially corrosive substances (OSHA regulation, 1910.133). However, even protective goggles are no match for chemicals under high pressure.
Diagnosis
History
The severity of ocular injury depends on 4 factors: the toxicity of the chemical, how long the chemical is in contact with the eye, the depth of penetration, and the area of involvement. It is therefore critical to take a careful history to document these factors. Patients should be asked when the injury occurred, whether they rinsed their eyes afterward and for how long, the mechanism of injury (Was the chemical under high pressure?), the type of chemical that splashed in the eye, and whether or not they were wearing eye protection. It is helpful to obtain the packaging of the chemical. There is often product information on this packaging, including chemical composition. If this information is not immediately available, chemical information can be found by contacting the local poison control center via AAPCC.org or 1-800-222-1222. However, irrigation when needed should not be delayed to obtain any of this history.
Physical Examination
Prior to a full ophthalmic examination, the pH of both eyes should be checked. If the pH is not in physiologic range, then the eye must be irrigated to bring the pH to an appropriate range (between 7 and 7.2). It is recommended to wait at least 5 minutes after irrigation before checking the pH to ensure that the pH does not rise or fall secondary to retained particulate matter.
The physical examination should be used to assess the extent and depth of injury (see classification schemes below). Specifically, the degree of corneal, conjunctival, and limbal involvement should be documented, as it can be used to predict ultimate visual outcome.[7]
The palpebral fissures should be checked, and the fornices should be swept during the initial examination. Both the palpebral and bulbar conjunctiva should be examined with fluorescein under a cobalt blue light. As above, retained particulate matter can cause persistent damage, despite irrigation. The intraocular pressure (IOP) should also be documented, as alkali injuries have been found to both acutely and chronically cause an elevation of IOP.[8]
Two major classification schemes for corneal burns are the Roper-Hall (modified Hughes) classification[9][10] and the Dua classification.[11] The Roper-Hall classification is based on the degree of corneal involvement and limbal ischemia. The Dua classification is based on an estimate of limbal involvement (in clock hours) and the percentage of conjunctival involvement. In a randomized controlled trial of acute burns, the Dua classification was found to be superior to the Roper-Hall in predicting outcome in severe burns.[7] However, both classification schemes are commonly employed in daily practice.
Symptoms
The most common symptoms are severe pain, epiphora, blepharospasm, and reduced visual acuity.
Management
Irrigation and Early Management
It is important to consider preophthalmologist management of chemical burns by bystanders, first responders, and emergency department (ED) physicians.
Irrigation is the cornerstone of managing chemical burns and should be initiated by bystanders and continued as transfer of care takes place between emergency medical services (EMS), ED physicians, and the ophthalmologist. Early irrigation is critical in limiting the duration of chemical exposure. The goal of irrigation is to remove the offending substance and restore the physiologic pH. It may be necessary to irrigate as much as 20 L to achieve this.
To position the patient for irrigation, the patient should ideally be seated upright with their head supported and tilted toward the affected side.[12] Irrigation may require manual opening of the eyelid of the affected eye to combat blepharospasm. The irrigating fluid should be administered nasal to lateral, poured away from the nonaffected eye to prevent injury to that eye.[12] Covering the noninjured eye with a shield may help prevent additional chemical injury. During irrigation, the patient should be asked to blink frequently.[13] Additionally, during irrigation, the patient should be asked to look in all directions to ensure that the conjunctival sacs are irrigated.[14][15]
Prior to beginning irrigation, disinfecting hands with an alcohol-based hand sanitizer is not recommended, because it could cause further irritation if it gets into the eye.[12] Rather, hand washing is preferable. Topical anesthetic such as lidocaine can be applied prior to irrigation to increase patient comfort. The patient’s eyelids may be held open manually or with a speculum. If available, devices such as the Morgan therapeutic lens, which can assist in eye irrigation, can be used. This lens is connected to intravenous (IV) tubing, which facilitates irrigation. However, it does not allow for removal of foreign particles from the eye. Additionally, the use of topical corticosteroids can treat acute inflammation due to chemical injury.[16]
Bystanders
In the event of a chemical injury, it is most important for bystanders and first responders to assess scene safety and take necessary precautions to avoid additional exposure or contamination.[17][18] After the scene is determined to be safe, any excess chemical present on the patient can be blotted.[18] Chemical burns must be managed with immediate removal of the offending agent and irrigation of the affected eye. Irrigation is crucial for reducing the time the eye is exposed to the chemical; however, it should be noted that irrigation is contraindicated in open-globe injuries.[19] Studies have shown that the severity of alkali burns and healing times were shortened with immediate copious irrigation.[20] Additionally, time to initial irrigation has the greatest influence on visual prognosis.[14][21]
Although use of a sterile irrigating fluid is preferable, bystanders should initiate irrigation of the affected eye immediately with any nontoxic liquid including tap water.[14][21] Use of alkaline or acidic solutions in an attempt to neutralize the chemical burn should be avoided, as it can result in further ocular damage or additional injury to surrounding areas of the body.[20]
Irrigation should ideally be initiated as quickly as possible and continue for as long as possible with several liters of fluid,[15][22] preferably until transfer of care takes place with first responders or the emergency department. During transfer of care, noting the offending chemical as well as how and when the injury occurred may aid in medical treatment. Locating the chemical container itself and handing it to emergency responders is one way to expedite this process.
EMS/First Responders
Emergency medical personnel should continue irrigation during transport. If available, sterile saline solution can be used to continue irrigation. Previous evidence suggests that eye irrigation should be prioritized over immediate comprehensive ocular assessment.[23] Irrigation of a chemical injury should take place for at least 20 minutes,[18] but in most cases it should continue until transfer of care to the emergency department (ED).
First responders should operate according to their level of training and certification, which may vary by state guidelines. In New York, Basic Emergency Medical Technicians (BLS) can assist with removal of contact lenses, if present, in the affected eye(s); presence of a contact lens can lead to persistence of the chemical burn, despite irrigation.[18] Paramedics (ALS) can instill 1 dose of topical anesthetic such as proparacaine HCl 0.5% solution or tetracaine HCl 0.5% solution, 1-2 gtts, topically, into the affected eye(s) to facilitate irrigation.[16]
ED Physician and Ophthalmologist
In a clinical setting, to optimize patient comfort and ensure effective delivery of the irrigating solution, a topical anesthetic is generally administered. An eyelid speculum or Morgan Lens (MorTan) can be used to keep the eye open, while the irrigating solution is delivered through IV tubing. While there is no widespread consensus on how long the eye should be irrigated, suggested guidelines recommend that irrigation should be continued for a minimum of 30 minutes using 1-3 L of fluid or continued until a physiological pH is reached.[24] The pH can be monitored using pH paper and should be measured at least 5 minutes after stopping irrigation.[15] Although irrigation is a crucial component of initial management, overirrigation can result in corneal edema.
It is also crucial to identify the presence of and remove caustic foreign bodies through visual inspection or by everting the eyelid. Particles lodged under lids or in the conjunctival fornices cause continued chemical exposure. Sterile cotton-tipped applicators wet with the irrigating fluid can be used to remove visible foreign particles, as well as to sweep the inferior and superior fornices.[12]
Types of Irrigating Fluid
A wide variety of solutions can be used for irrigation, each with different benefits. However, regardless of the type of irrigating fluid used, not delaying irrigation is paramount to limiting the duration of chemical exposure and thus minimizing ocular damage and restoring visual function.
Tap water is widely available but is not sterile and is hypotonic to the corneal stroma. Use of hypotonic solutions increases water influx into the cornea, leading to further diffusion of corrosive material into the eye and increased corneal edema.[14][25] Other readily available solutions in the ED include normal saline, lactated Ringer’s IV solution, phosphate buffer solution, and balanced saline solution (BSS), which are isotonic to the corneal stroma and may be superior to tap water, since they are sterile.[14] Phosphate buffer is also at physiological pH and can be used to correct pH abnormalities from the offending substance. A study by Herr and colleagues compared normal saline (NS), normal saline with bicarbonate (NS + Bicarb), lactated Ringer’s solution (LR), and BSS Plus (Alcon Laboratories) to investigate which solution optimized patient comfort. They found that patients tolerated and preferred BSS irrigation compared to NS, NS + Bicarb, and LR.[26]
Use of hypertonic solutions is preferable because they increase the osmotic pressure to mobilize water and dissolved corrosives out of corneal tissue.[14] Thus, they prevent further uptake of the corrosive chemical into the cornea, leading to a reduction in corneal swelling.[25] In experiments in rabbit eyes following sodium hydroxide injury, a borate buffer solution called Cederroth eye wash (Cederroth Industrial Products) and a diphoterine and Previn solution (Prevor) more efficiently normalized the pH than saline and phosphate buffer solutions.[27] Diphoterine is a hypertonic amphoteric solution that can be used in both alkali and acid burns. It is widely used in Europe as a first-line agent for chemical burns and has been shown to improve healing time and reduce the intensity of the pain.[28] Because diphoterine is amphoteric, it is able to quickly neutralize the corneal stroma to physiologic pH; for a given amount of diphoterine, 17x the amount of volume in water would be needed to neutralize the pH.[29] It has a buffering capacity similar to phosphate buffer.[29] Additionally, diphoterine has been shown to lead to faster re-epithelialization of the stroma and to limit tissue damage, pain, and inflammation.[23][30] While diphoterine may be the irrigating fluid of choice, immediate irrigation should not be delayed if diphoterine is not readily available.
Medical Therapy
Patients with mild to moderate injury (grades I and II) have a good prognosis and the injury can often be managed successfully with medical treatment alone. The aims of medical treatment are to enhance recovery of the corneal epithelium and augment collagen synthesis, while also minimizing collagen breakdown and controlling inflammation.[3]
Standard Treatments
Antibiotics: A topical antibiotic ointment such as erythromycin ointment 4 times daily can be used to provide ocular lubrication and prevent superinfection. Stronger antibiotics (eg, a topical fluoroquinolone) are employed for more severe injuries (eg, grades II and above).
Cycloplegic agents: Agents such as atropine or cyclopentolate can help with comfort.
Artificial tears: These and other lubricating eyedrops, preferably preservative free, should be used generously for comfort.
Steroid drops: In the first week following injury, topical steroids can help calm inflammation and prevent further corneal breakdown.[31] In mild injuries, topical prednisolone (Pred Forte) can be employed 4 times daily. In more severe injuries, prednisolone can be used every hour. After about 1 week of intensive steroid use, the steroids should be tapered because the balance of collagen synthesis versus collagen breakdown may tip unfavorably toward collagen breakdown.[32]
Other Treatments
Ascorbic acid: This cofactor in collagen synthesis may be depleted following chemical injury. Ascorbic acid can be used as a topical drop (10% every hour) or orally (2 grams 4 times daily in adults). In one study, severe alkali burns in rabbit eyes were associated with reduced ascorbic acid levels in the aqueous humor. This reduction correlated with corneal stromal ulceration and perforation. Systemic administration of vitamin C helped promote collagen synthesis and reduce the level of ulceration.[33] Care must be taken in patients with compromised renal function because high levels of vitamin C are potentially toxic to the kidneys.[34]
Doxycycline: Doxycycline acts independently of its antimicrobial properties to reduce the effects of matrix metalloproteinases (MMPs), which can degrade type I collagen. The tetracycline class inhibits MMPs by restriction of the gene expression of neutrophil collagenase and epithelial gelatinase, suppression of alpha1-antitrypsin degradation, and scavenging of reactive oxygen species, thereby reducing ocular surface inflammation.[35][36] Doxycycline should be used with caution in children and females of childbearing age.
Citrate drops: Histological sections of cornea from alkali burns reveal an intense polymorphonuclear infiltrate (PMN).[37] PMNs provide a major source of proteolytic enzymes, which can dissolve the corneal stromal collagen. Deficiency in calcium inhibits the PMNs from granulating and releasing proteolytic enzymes. Citrate is a potent chelator and can therefore decrease proteolytic activity. Citrate also appears to inhibit collagenases.[38][39]
1% medroxyprogesterone: This progestational steroid has less anti-inflammatory potency than corticosteroids, but it has a minimum effect on stromal repair. Medroxyprogesterone can therefore be substituted for cortical steroids after 10-14 days of steroid treatment.[2][40]
Platelet-rich plasma eyedrops: These have been found to be rich in growth factors, and platelet-rich plasma eyedrops can lead to faster epithelialization for certain classes of burns.[41]
Surgical Treatments
Debridement of necrotic epithelium: Debridement should be performed as early as possible because necrotic tissue serves as a source of inflammation and can inhibit epithelialization.[3]
Conjunctival/Tenon transposition (tenonplasty): In grade IV burns, anterior segment necrosis can result from loss of limbal vascular blood supply. In severe limbal ischemia, a sterile corneal ulceration can ensue. After removal of necrotic tissue, a tenonplasty (advancement of the conjunctiva and Tenon tissue to the limbus) can be employed to reestablish limbal vascularity and facilitate re-epithelialization.[42]
Amniotic membrane transplantation (AMT): The purpose of AMT is to rapidly restore the conjunctival surface and to reduce limbal and stromal inflammation. The benefits are thought to be twofold: physical and biological. Physically, AMT has been shown to improve patient comfort by reduction of eyelid friction. Numerous studies have found a reduction in pain following AMT for moderate to severe burns.[43][44] Through its physical actions, AMT may also prevent symblepharon formation. Amniotic membrane is also felt to have biologic effects.[45] It expresses TGF-β1 and epidermal growth factor, which have roles in wound healing.[46][47] It has also been found to have anti-inflammatory properties.[48][49][50] Taken together, these biological effects may dampen inflammation, promote epithelial growth, prevent scarring, and prevent neovascularization. New delivery devices like Prokera (BioTissue), which consists of a piece of cryopreserved amniotic membrane clipped into a dual-ring system, like a symblepharon ring, allow rapid and sutureless placement of amniotic membrane.[51] A Cochrane review found only 1 randomized controlled trial of amniotic membrane for treatment of chemical ocular burn in the first 7 days following injury.[1] Patients with moderate burns were found to have a significantly better visual acuity following AMT compared to medical therapy alone.[52] However, this was an unmasked trial, and there were uneven baseline characteristics of the control and treatment eyes.[1] While case series and reviews show great promise of AMT in the treatment of chemical burns, conclusive evidence is still lacking.
Limbal stem cell transplantation: Much of the damage following chemical injuries results from limbal ischemia and the subsequent loss of stem cells capable of repopulating the corneal epithelium. Limbal stem cell transplants have been employed to replace this critical group of cells. Limbal stem cells are located at the base of the limbal epithelium and are responsible for repopulation of cells in the corneal epithelium and inhibition of conjunctival growth over the cornea.[53] Limbal autografts can be used from the healthy contralateral eye if only one eye is injured in a chemical burn, typically after the acute phase of injury has passed.[54] When both eyes are injured, transplants have been attempted from living related donors. In a study from China, a portion of the limbus of HLA-matched living related donors (allograft) was transplanted following chemical injury. Patients experienced a reduction in vascularity, improved corneal opacity, and corneal epithelialization without the need for systemic immunosuppression.[53] Another option is to use cadaveric donors. This requires systemic immunosuppression.[55] When possible, limbal stem cell transplantation should be delayed until ocular surface inflammation has quieted.[56][57]
Cultivated oral mucosal epithelial transplantation (COMET): COMET can also be used to promote re-epithelialization and reduce inflammation in corneal burns. The cells are harvested from the patient’s own buccal mucosa so that systemic immunosuppression is not necessary.[58][59]
Boston Keratoprosthesis: Severe chemical injury leads to chronic inflammation and scarring, making visual recovery challenging. In cases with severe inflammation, limbal stem cell transplants and corneal transplants do not survive. In these most difficult cases, the Boston Keratoprosthesis can be used. Because it is independent of stem cell function, it does not require systemic immunosuppression.[60]
Recommended Treatment
While there is variability in treatment strategies of chemical burns, most authors recommended a graded approach depending on the severity of injury. Mild burns (Roper-Hall grade I) respond well to medical treatments and lubrication, while more severe burns necessitate more intensive medical therapies and surgery. Below is a paradigm for the initial treatment of chemical injury based on the Roper-Hall grade of injury.[3][61]
Grade I
- Topical antibiotic ointment (erythromycin ointment or similar) 4 times a day
- Prednisolone acetate 1% 4 times a day
- Preservative-free artificial tears as needed
- If there is pain, consider a short-acting cycloplegic like cyclopentolate 3 times a day.
Grade II
- Topical antibiotic drop like fluoroquinolone 4 times daily
- Prednisolone acetate 1% hourly while awake for the first 7-10 days. Consider tapering the steroid if the epithelium has not healed by day 10-14. If an epithelial defect persists after day 10, consider progestational steroids (1% medroxyprogesterone 4 times daily).
- Long-acting cycloplegic like atropine
- Oral vitamin C, 2 grams 4 times a day
- Doxycycline, 100 mg twice a day (avoid in children)
- Sodium ascorbate drops (10%) hourly while awake
- Preservative-free artificial tears as needed
- Debridement of necrotic epithelium and application of tissue adhesive as needed
Grade III
- As for grade II
- Consider AMT/Prokera placement. This should ideally be performed in the first week of injury. Experienced surgeons have emphasized placement of the amniotic membrane to cover the palpebral conjunctiva by suturing to the lids in the operating room, not just covering the cornea and bulbar conjunctiva.
Grade IV
- As for grade II/III
- Early surgery is usually necessary. For significant necrosis, a tenonplasty can help reestablish limbal vascularity. An amniotic membrane transplant is often necessary due to the severity of the ocular surface damage.
Stages of Ocular Recovery
Stages of ocular recovery following chemical injury are described below.[3][6]
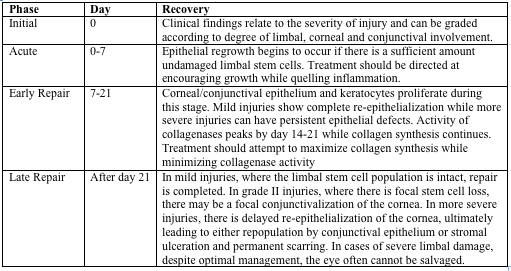
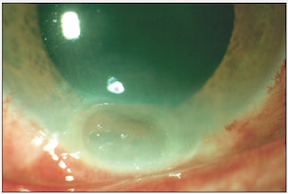
Grade II burn (above), about 1 month following injury. There is focal thinning. The cornea was treated with tissue adhesive and a bandage contact lens.
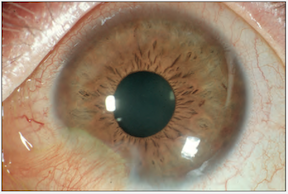
The final result of this grade II burn (above). Focal conjunctivalization.
*Images courtesy of Dr. Kathryn Colby (Massachusetts Eye and Ear Infirmary)
Follow-up
With severe chemical burns, patients should initially be followed daily. If there is concern for compliance with medication or if the patient is a child, one should consider inpatient admission. Once the health of the ocular surface has been restored, follow-up can be spread apart. However, even in the healthiest-appearing eyes, patients need long-term monitoring for glaucoma and dry eye, as described below.
Other Long-Term Complications
Glaucoma
Glaucoma is quite common following ocular injury, ranging in frequency from 15%-55% in patients with severe burns.[8] The mechanism of glaucoma is multifactorial and includes contraction of the anterior structures of the globe secondary to chemical and inflammatory damage, inflammatory debris in the trabecular meshwork, and damage to the trabecular meshwork itself.[62] More severe burns (Roper-Hall grade III or IV) have been found to have significantly higher IOP at presentation and were more likely to require long-term glaucoma medication and undergo glaucoma surgery than grade I or II injuries.[8] Glaucoma medications should be prescribed as necessary to maintain normal IOP.
Dry Eye
Chemical injury can destroy conjunctival goblet cells, leading to a reduction or even absence of mucus in the tear film, and compromising the proper dispersion of the precorneal tear film. This mucus deficiency results in keratoconjunctivitis sicca (dry eye).[63] Even in well-healed eyes, chronic dry eye can cause significant morbidity because of discomfort, visual disturbance, and potential for damage of the ocular surface.
Damage to Eyelids or Palpebral Conjunctiva
Direct chemical damage to the conjunctiva can lead to scarring, forniceal shortening, symblepharon formation, and cicatricial entropion or ectropion. These entities are encountered weeks to months after injury and can be treated by suppressing inflammation and with early amniotic membrane transplantation or oral mucosal graft.[3][64][65]
Additional Resources
- American Association of Poison Control Centers: http://www.aapcc.org (1-800-222-1222)
- Iowa State University’s Safety Data Sheets (SDSs): https://www.ehs.iastate.edu/sds
- Occupational Safety and Health Administration requirement for eye protection at work: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=9778
- Amniograft & Prokera: http://www.biotissue.com/
References
- ↑ 1.0 1.1 1.2 Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012;2012(9):CD009379.
- ↑ 2.0 2.1 2.2 Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41(4):275-313.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Colby K. Chemical injuries of the cornea. Focal Points. American Academy of Ophthalmology. 2010;28(1):1-14.
- ↑ Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Curr Opin Ophthalmol. 2010;21(4):317-321.
- ↑ 5.0 5.1 Barouch F, Colby KA. Evaluation and initial management of patients with ocular and adnexal trauma. In: Miller JW, Azar DT, Blodi B, eds. Albert and Jakobiec's Principles and Practice of Ophthalmology. 3rd ed. WB Saunders Elsevier; 2008:5071-5092.
- ↑ 6.0 6.1 McCulley J. Chemical injuries. In: Smolin G, Thoft RA, eds. The Cornea: Scientific Foundation and Clinical Practice. 2nd ed. Little Brown and Co; 1987.
- ↑ 7.0 7.1 Gupta N, Kalaivani M, Tandon R. Comparison of prognostic value of Roper Hall and Dua classification systems in acute ocular burns. Br J Ophthalmol. 2011;95(2):194-198.
- ↑ 8.0 8.1 8.2 Lin MP, Ekşioğlu Ü, Mudumbai RC, Slabaugh MA, Chen PP. Glaucoma in patients with ocular chemical burns. Am J Ophthalmol. 2012;154(3):481-485.e1.
- ↑ Hughes WF Jr. Alkali burns of the eye; review of the literature and summary of present knowledge. Arch Ophthalmol. 1946;35:423.
- ↑ Roper-Hall MJ. Thermal and chemical burns. Trans Ophthalmol Soc UK (1962). 1965;85:631-653.
- ↑ Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. Br J Ophthalmol. 2001;85(11):1379-1383.
- ↑ 12.0 12.1 12.2 12.3 Marsden J. How to perform irrigation of the eye. Nurs Stand. 2016;30(23):36-39.
- ↑ Statewide Pre-Hospital Treatment Protocols Version 16.04. New York State Bureau of Emergency Medical Services: New York State Department of Health; 2019.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 Kuckelkorn R, Schrage N, Keller G, Redbrake C. Emergency treatment of chemical and thermal eye burns. Acta Ophthalmol Scand. 2002;80(1):4-10.
- ↑ 15.0 15.1 15.2 Scott R. The injured eye. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):251-260.
- ↑ 16.0 16.1 Advanced Life Support (Paramedic) Protocols, in Prehospital Treatment Protocols. The Regional Emergency Medical Advisory Committee of New York City; 2019.
- ↑ Statewide Pre-Hospital Treatment Protocols Version 16.04. New York State Bureau of Emergency Medical Services: New York State Department of Health; 2019.
- ↑ 18.0 18.1 18.2 18.3 Basic Life Support Protocols, in Prehospital Treatment Protocols. The Regional Emergency Medical Advisory Committee of New York City; 2019.
- ↑ Eye Trauma: Initial Care (CPG ID:03), in Joint Trauma System Clinical Practice Guideline. Department of Defense Center of Excellence for Trauma: Joint Trauma System; 2019.
- ↑ 20.0 20.1 Ikeda N, Hayasaka S, Hayasaka Y, Watanabe K. Alkali burns of the eye: effect of immediate copious irrigation with tap water on their severity. Ophthalmologica. 2006;220(4):225-228.
- ↑ 21.0 21.1 Burns FR, Paterson CA. Prompt irrigation of chemical eye injuries may avert severe damage. Occup Health Saf. 1989;58(4):33-36.
- ↑ Khaw PT, Shah P, Elkington AR. Injury to the eye. BMJ. 2004;328(7430):36.
- ↑ 23.0 23.1 Chau JPC, Lee DTF, Lo SHS. A systematic review of methods of eye irrigation for adults and children with ocular chemical burns. Worldviews Evid Based Nurs. 2012;9:129-138.
- ↑ Sharma N, Kaur M, Agarwal T, Sangwan VS, Vajpayee RB. Treatment of acute ocular chemical burns. Surv Ophthalmol. 2018;63(2):214-235.
- ↑ 25.0 25.1 Baradaran-Rafii A, Eslani M, Haq Z, Shirzadeh E, Huvard MJ, Djalilian AR. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf. 2017;15(1):48-64.
- ↑ Herr RD, White GL Jr, Bernhisel K, Mamalis N, Swanson E. Clinical comparison of ocular irrigation fluids following chemical injury. Am J Emerg Med. 1991;9(3):228-231.
- ↑ Rihawi S, Frentz M, Schrage NF. Emergency treatment of eye burns: which rinsing solution should we choose? Graefes Arch Clin Exp Ophthalmol. 2006;244(7):845-854.
- ↑ Lynn DD, Zukin LM, Dellavalle R. The safety and efficacy of Diphoterine for ocular and cutaneous burns in humans. Cutan Ocul Toxicol. 2017;36(2):185-192.
- ↑ 29.0 29.1 Alexander KS, Wasiak J, Cleland H. Chemical burns: Diphoterine untangled. Burns. 2018;44(4):752-766.
- ↑ Fortin JL, Fontaine M, Bodson L, et al. Use of an amphoteric solution in eye, skin and oral chemical exposures: retrospective multicenter clinical case series. Ferment Technol. 2017;07.
- ↑ Dohlman CH, Cade F, Pfister R. Chemical burns to the eye: paradigm shifts in treatment. Cornea. 2011;30(6):613-614.
- ↑ Donshik PC, Berman MB, Dohlman CH, Gage J, Rose J. Effect of topical corticosteroids on ulceration in alkali-burned corneas. Arch Ophthalmol. 1978;96(11):2117-2120.
- ↑ Pfister RR, Haddox JL, Yuille-Barr D. The combined effect of citrate/ascorbate treatment in alkali-injured rabbit eyes. Cornea. 1991;10(2):100-104.
- ↑ Gabardi S, Munz K, Ulbricht C. A review of dietary supplement-induced renal dysfunction. Clin J Am Soc Nephrol. 2007;2(4):757-765.
- ↑ Ralph RA. Tetracyclines and the treatment of corneal stromal ulceration: a review. Cornea. 2000;19(3):274-277.
- ↑ Smith VA, Cook SD. Doxycycline--a role in ocular surface repair. Br J Ophthalmol. 2004;88(5):619-625.
- ↑ Matsuda H, Smelser GK. Epithelium and stroma in alkali-burned corneas. Arch Ophthalmol. 1973;89(5):396-401.
- ↑ Haddox JL, Pfister RR, Slaughter SE. An excess of topical calcium and magnesium reverses the therapeutic effect of citrate on the development of corneal ulcers after alkali injury. Cornea. 1996;15(2):191-195.
- ↑ Pfister RR, Haddox JL, Sommers CI. Effect of synthetic metalloproteinase inhibitor or citrate on neutrophil chemotaxis and the respiratory burst. Invest Ophthalmol Vis Sci. 1997;38(7):1340-1349.
- ↑ Gross J, Azizkhan RG, Biswas C, Bruns RR, Hsieh DS, Folkman J. Inhibition of tumor growth, vascularization, and collagenolysis in the rabbit cornea by medroxyprogesterone. Proc Natl Acad Sci USA. 1981;78(2):1176-1180.
- ↑ Panda A, Jain M, Vanathi M, Velpandian T, Khokhar S, Dada T. Topical autologous platelet-rich plasma eyedrops for acute corneal chemical injury. Cornea. 2012;31(9):989-993.
- ↑ Kuckelkorn RN, Schrage N, Reim M. Treatment of severe eye burns by tenonplasty. Lancet. 1995;345(8950):657-658.
- ↑ Shafto CM. A simple method of inserting amniotic membrane grafts into the conjunctival sac. Br J Ophthalmol. 1950;34(7):445-446.
- ↑ Baum J. Thygeson lecture. Amniotic membrane transplantation: why is it effective? Cornea. 2002;21(4):339-341.
- ↑ Tseng SCG, Di Pascuale MA, Liu D, Gao YY, Baradaran-Rafii A. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology. 2005;112(5):896-903.
- ↑ Hopkinson A, McIntosh RS, Tighe PJ, James DK, Dua HS. Amniotic membrane for ocular surface reconstruction: donor variations and the effect of handling on TGF-beta content. Invest Ophthalmol Vis Sci. 2006;47(10):4316-4322.
- ↑ Gicquel J-J, Dua HS, Brodie A, et al. Epidermal growth factor variations in amniotic membrane used for ex vivo tissue constructs. Tissue Eng Part A. 2009;15(8):1919-1927.
- ↑ Ueta M, Kweon M-N, Sano Y, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol. 2002;129(3):464-470.
- ↑ Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348-352.
- ↑ Li W, He H, Kawakita T, Espana EM, Tseng SCG. Amniotic membrane induces apoptosis of interferon-gamma activated macrophages in vitro. Exp Eye Res. 2006;82(2):282-292.
- ↑ Kheirkhah A, Johnson DA, Paranjpe DR, Raju VK, Casas V, Tseng SCG. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch Ophthalmol. 2008;126(8):1059-1066.
- ↑ Tandon R, Gupta N, Kalaivani M, Sharma N, Titiyal JS, Vajpayee RB. Amniotic membrane transplantation as an adjunct to medical therapy in acute ocular burns. Br J Ophthalmol. 2011;95(2):199-204.
- ↑ 53.0 53.1 Huang T, Wang Y, Zhang H, Gao N, Hu A. Limbal from living-related donors to treat partial limbal deficiency secondary to ocular chemical burns. Arch Ophthalmol. 2011;129(10):1267-1273.
- ↑ Morgan S, Murray A. Limbal autotransplantation in the acute and chronic phases of severe chemical injuries. Eye (Lond). 1996;10(Pt 3):349-354.
- ↑ Tsai RJ, Tseng SC. Human allograft limbal transplantation for corneal surface reconstruction. Cornea. 1994;13(5):389-400.
- ↑ Samson CM, Nduaguba C, Baltatzis S, Foster CS. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology. 2002;109(5):862-868.
- ↑ Liang L, Sheha H, Tseng SCG. Long-term outcomes of keratolimbal allograft for total limbal stem cell deficiency using combined immunosuppressive agents and correction of ocular surface deficits. Arch Ophthalmol. 2009;127(11):1428-1434.
- ↑ Ma D, Kuo M-T, Tsai Y-J, et al. Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye (Lond). 2009;23(6):1442-1450.
- ↑ Nakamura T, Inatomi T, Sotozono C, Amemiya T, Kanamura N, Kinoshita S. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88(10):1280-1284.
- ↑ Khan B, Dudenhoefer EJ, Dohlman CH. Keratoprosthesis: an update. Curr Opin Ophthalmol. 2001;12(4):282-287.
- ↑ Hemmati HD, Colby KA. Treating acute chemical injuries of the cornea. EyeNet. October 1, 2012:43-45.
- ↑ Paterson CA, Pfister RR. Intraocular pressure changes after alkali burns. Arch Ophthalmol. 1974;91(3):211-218.
- ↑ Le Q, Chen Y, Wang X, Li Y, Hong J, Xu J. Vision-related quality of life in patients with ocular chemical burns. Invest Ophthalmol Vis Sci. 2011;52(12):8951-8956.
- ↑ Kheirkhah A, Ghaffari R, Kaghazkanani R, Hashemi H, Behrouz MJ, Raju VK. A combined approach of amniotic membrane and oral mucosa transplantation for fornix reconstruction in severe symblepharon. Cornea. 2013;32(2):155-160.
- ↑ Tuft SJ, Shortt AJ. Surgical rehabilitation following severe ocular burns. Eye (Lond). 2009;23(10):1966-1971.