Neovascular Glaucoma
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Neovascular glaucoma (NVG) is a severe form of secondary glaucoma characterized by proliferation of fibrovascular tissue in the anterior chamber angle.[1] Since Coats first noted, in 1906, new vessel formation on the iris (rubeosis iridis) in eyes with central retinal vein occlusion, this condition has been noted previously by names including hemorrhagic glaucoma, congestive glaucoma, thrombotic glaucoma, and rubeotic glaucoma.[2] Weiss and colleagues introduced the term neovascular glaucoma in 1963.[3] The common denominator predisposing to this condition is usually retinal ischemia, although some cases are associated with other ocular or extraocular entities. Neovascular glaucoma runs an aggressive clinical course and the condition is usually refractory to medical therapy alone. Surgical approaches to managing this complicated form of glaucoma have evolved over the past few decades while often still resulting in a guarded visual prognosis. In light of the association of vascular endothelial growth factor (VEGF) with retinal ischemia, the advent of anti-VEGF drugs provides a welcome addition in the treatment strategy for this potentially devastating condition.[4][5][6]
Disease Entity
Neovascular Glaucoma
Disease
Neovascular glaucoma (NVG) can present through either a secondary open-angle or secondary closed-angle mechanism, depending on the extent and duration of neovascularization. Neovascularization within any organ system can be characterized as the growth of vessels into or onto tissues not normally vascularized. Blood vessels generated through neovascularization have different qualities than normal ones. The walls of these vessels have increased permeability due to the absence of tight intercellular junctions, which are prone to vascular leakage and variable amounts of cellular inflammation.[7] When new blood vessels appear within the anterior chamber angle, aqueous outflow can be compromised with extension of these new vessels across the scleral spur and subsequent obstruction of the trabecular meshwork. The new blood vessels are usually accompanied by a fibrous membrane, which, in addition to obstructing aqueous outflow, contracts to result in formation of peripheral anterior synechiae and progressive angle closure.
Etiology
Rubeosis iridis and NVG have been associated with a wide range of conditions (see Risk Factors). Of these conditions, retinal ischemia accounts for the majority of the causes, with central retinal vein occlusion (CRVO) and diabetes retinopathy underlying nearly two-thirds of all NVG cases. Retinal ischemia triggers a cascade of events, beginning with an inadequate oxygen supply to the retinal cells, leading to the release of various angiogenic factors including VEGF and interleukin-6. Normally VEGF levels are in equilibrium with pigment epithelium-derived growth factor (PEDF), an antiangiogenic factor. When the equilibrium between VEGF and PEDF is shifted in favor of VEGF, this promotes activation, proliferation, and migration of endothelial cells, leading to neovascularization of the anterior segment. Increased levels of Interleukin-6 have also been noted in the aqueous humor of patients with neovascular glaucoma due to central retinal vein occlusion.[8]
Risk factors
Retinal Ischemia, Irradiation, Tumors, Ocular Inflammatory Diseases, Surgical Causes, Systemic Vascular Disorders
General pathology
Normal blood vessels in the iris have non-fenestrated endothelial cells with tight intercellular junctions. New vessels in NVG are thin walled, lacking a muscular layer with little adventitia or supporting tissue. Neovascular vessels demonstrate gaps in the endothelial cells on electron microscopy, fenestrations in the endothelial cells, and basement membrane changes in new vessels in diabetic eyes.[9][10] The new vessels are often accompanied by a fibrovascular membrane consisting of proliferating myofibroblasts, which are fibroblasts with smooth muscle differentiation.[11] Scanning electron microscopy has confirmed the presence of this membrane, and the contractile smooth muscle components can lead to anatomical changes including effacement of the iris surface, ectropion uveae, peripheral anterior synechiae formation, and angle closure.[11]
The American Academy of Ophthalmology's Pathology Atlas[12] contains a virtual microscopy image of neovascular glaucoma.
Clinical course
The 3 most common clinical entities leading to rubeosis iridis are diabetes mellitus, CRVO, and carotid occlusive disease. Patients with these conditions or any other predisposing factors should undergo careful slit-lamp examination to check for early signs of neovascularization. Fundus fluorescein angiography or quantitative laser photometry with iris fluorescein angiography may aid in detecting occult neovascularization. The progression of rubeosis iridis to advanced NVG can be broken down into 4 stages: pre-rubeosis, rubeosis, open-angle glaucoma, and angle-closure glaucoma. In the pre-rubeosis stage, new vessels on the iris or in the angle are not visible. In the rubeosis stage, tiny tufts of new vessels begin to appear on the iris, usually beginning at the papillary margin. The angle is relatively spared and the intraocular pressure is normal at this stage. In the third, or open-angle, glaucoma stage, new vessels begin to invade the iris stroma and the anterior chamber angle. The angle will appear open on gonioscopy, while the intraocular pressure may be either normal or elevated. In the final stage, or angle-closure glaucoma stage, the new vessels become more widespread, with an overlying fibrovascular membrane resulting in flattening of the iris and anterior displacement of the iris, resulting in progressively synechial closure of the drainage angle.
Primary prevention
Awareness of risk factors and early detection of retinal ischemia can minimize subsequent anterior segment neovascularization or expedite treatment leading to vessel remission.
Diagnosis
Diagnosis of NVG is primarily clinical. Early neovascularization of the angle (NVA) can be difficult to visualize without using a viscous coupling agent (eg, hypromellose ophthalmic solution), along with the Goldmann gonioscope.
History
In patients with diabetes, onset of NVG usually correlates with glucose control. The Diabetes Control Complications Trial (DCCT) noted a 24% incidence of NVG over 9 years amongst the standard treatment group, compared to 8% in the intensively treated group.[13] Presence of proliferative diabetic retinopathy and anterior segment neovascularization should strongly suggest diabetic NVG, whereas painless sudden decrease in vision 60-90 days prior to presentation would be typical of CRVO-related NVG. History of significant carotid artery occlusion on the same side with elevated intraocular pressure would raise one’s suspicion of ocular ischemic syndrome as the etiology of NVG.
Signs
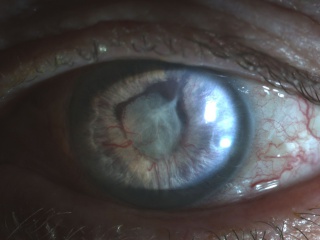
The earliest signs of NVG are tiny tufts of new vessels at the margin of the pupil and engorgement of major arterial circle of the iris. Whereas normal vessels tend to lie in the stroma and are radial in orientation, neovascular vessels appear on the surface of the iris and take on an irregular, meandering pattern.[14] As the vessels migrate toward the angle, one may observe on gonioscopy very fine arborized vessels, which cross over the scleral spur onto the trabecular meshwork, whereas normal vessels typically remain behind the scleral spur.[15] With advancing disease, a fibrovascular membrane, invisible on gonioscopy, has a tendency to contract and pull the vessels taut.
These radial traction forces have 2 main consequences:
- Migration of the posterior pigmented iris onto the anterior surface along the papillary margin, in addition to effacement of the normal iris surface architecture, resulting in a smooth iris.
- Tenting of iris onto trabecular meshwork, with a cascade of coalescing peripheral anterior synechiae leading to synechial angle closure.[14]
Photographs courtesy of Sarwat Salim, MD, University of Tennessee
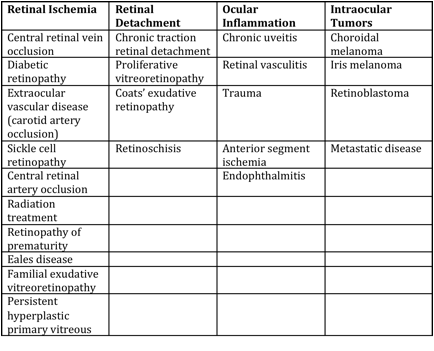
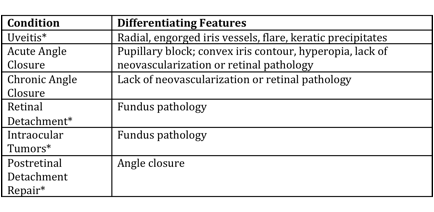
Differential diagnosis
Differential diagnosis can be broken down into etiologies of true NVG and other ocular conditions that can clinically mimic the presentation of NVG. These are summarized respectively in the tables below (from Glaucoma: Science and Practice by John Morrison and Irvin Pollack).
Mechanisms of Anterior Segment Neovascularization and Predisposing Conditions[16]
Differential Diagnosis of Neovascular Glaucoma[16]
General treatment
Adequate treatment of retinal ischemia with panretinal photocoagulation (PRP) is essential in reducing the stimulus for neovascularization of the anterior segment, which may prevent the need for additional surgery. Treatment of underlying systemic disease may improve neovascularization of the iris, as is the case with endarterectomy for carotid occlusive disease and ocular ischemia. The advent of anti-VEGF agents has led to their use in the form of intravitreal injections prior to PRP and/or surgical control of intraocular pressure (IOP). The duration of suppression of iris and angle neovascularization lasts approximately 3-6 weeks with anti-VEGF injections, thereby creating a window of opportunity to allow adequate PRP and/or glaucoma surgery to be carried out.[17][18]
Medical therapy
Selection of specific agents to lower IOP depends upon the stage of NVG. Because elevated IOP is in part due to compromised trabecular meshwork function, aqueous suppressants (beta blockers, alpha-agonists, carbonic anhydrase inhibitors) should theoretically have the greatest efficacy. Prostaglandins may also be effective, and while a theoretical risk for exacerbating ocular inflammation exists, no evidence to date supports this in NVG. Because aqueous suppressants are often insufficient to achieve IOP control, prostaglandin analogues should be used as needed. Miotic agents or any other medications acting on aqueous outflow through the conventional pathway are least likely to be effective if the angle is already closed. Osmotic agents may be used to clear the cornea to improve visualization for treatment or diagnosis. Adjunctive treatment with a short course of topical steroids may control inflammation to improve outcome of subsequent surgery, and cycloplegics may aid in patient comfort and improve visibility for PRP.
Surgery
In eyes with vision better than 20/400, most glaucoma specialists prefer glaucoma drainage implant placement or filtering surgery versus cyclophotocoagulation. Regardless of the surgical procedure chosen, preoperative panretinal photocoagulation should be performed whenever possible.[19] [20] [21]
The presence of florid neovascularization poses a significant risk for intraoperative and postoperative hemorrhage, either extra- or intraocular.[17] While trabeculectomy offers the advantage of achieving lower postoperative IOP compared to aqueous shunts, failure to adequately resolve active neovascularization will lead to bleb failure through conjunctival scarring at the filtration site. Aqueous shunts bear the advantage of avoiding the necessity of surgical iridectomy, thereby decreasing the risk of intraoperative hemorrhage. In addition, aqueous shunts are more tolerant of intraoperative and postoperative bleeding and postoperative fibrin reaction. Therefore, in the setting of active neovascularization, inflammation, and/or hyphema, an aqueous shunt is preferred. In a quiet eye with a view for adequate PRP, trabeculectomy may be considered. More recently, adjunct treatment with anti-VEGF agents has also shown promise for decreasing intraoperative hemorrhage while improving IOP control and surgical success.[6][22][23] Cyclophotocoagulation is reserved for eyes with poor visual prognosis (20/400 or worse) or patients who are poor surgical candidates. Transscleral diode and YAG techniques are less likely to result in hypotony and phthisis compared to cryotherapy.[24] [25] [26]
Prognosis
The most successful NVG treatment is preventing neovascularization from occurring in the first place. A high index of suspicion based on the patient’s medical history and early recognition of eyes at risk for NVG is crucial to a favorable long-term outcome.
References
- ↑ Wand M. Neovascular glaucoma. In: Ritch R, Shields MB, Krupin T, eds. The Glaucomas: Clinical Science. 2nd. Ed. St. Louis: Mosby; 1996:1073-1129. Ch 51.
- ↑ Coats G., Further cases of thrombosis of the central vein. Roy Lond Ophthal Hosp Rep. 1906; 16:516.
- ↑ WEISS DI, SHAFFER RN, NEHRENBERG TR. Neovascular gluacoma complicating carotid-cavernous fistula. Arch Ophthalmol. 1963 Mar;69:304-7. doi: 10.1001/archopht.1963.00960040310007. PMID: 13999715.
- ↑ Sivak-Callcott JA, O'Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001 Oct;108(10):1767-76; quiz1777, 1800. doi: 10.1016/s0161-6420(01)00775-8. PMID: 11581047.
- ↑ Hayreh SS. Neovascular glaucoma. Prog Retin Eye Res. 2007 Sep;26(5):470-85. doi: 10.1016/j.preteyeres.2007.06.001. Epub 2007 Aug 8. PMID: 17690002; PMCID: PMC2871536.
- ↑ 6.0 6.1 Simha A, Aziz K, Braganza A, Abraham L, Samuel P, Lindsley KB. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev. 2020 Feb 6;2(2):CD007920. doi: 10.1002/14651858.CD007920.pub3. Update in: Cochrane Database Syst Rev. 2023 Apr 3;4:CD007920. doi: 10.1002/14651858.CD007920.pub4. PMID: 32027392; PMCID: PMC7003996.
- ↑ Tamura T. [Electron microscopic study on the small blood vessels in rubeosis iridis diabetica]. Nippon Ganka Gakkai Zasshi. 1968 Nov;72(11):2340-52. Japanese. PMID: 5752171.
- ↑ Chen KH, Wu CC, Roy S, Lee SM, Liu JH. Increased interleukin-6 in aqueous humor of neovascular glaucoma. Invest Ophthalmol Vis Sci. 1999 Oct;40(11):2627-32. PMID: 10509659.
- ↑ Tamura T. [Electron microscopic study on the small blood vessels in rubeosis iridis diabetica]. Nippon Ganka Gakkai Zasshi. 1968 Nov;72(11):2340-52. Japanese. PMID: 5752171.
- ↑ Vannas A. Fluorescein angiography of the vessels of the iris in pseudoexfoliation of the lens capsule, capsular glaucoma and some other forms of glaucoma. Acta Ophthalmol Suppl. 1969;105:1-75. PMID: 4313415.
- ↑ 11.0 11.1 John T, Sassani JW, Eagle RC Jr. The myofibroblastic component of rubeosis iridis. Ophthalmology. 1983 Jun;90(6):721-8. doi: 10.1016/s0161-6420(83)34520-6. PMID: 6193475.
- ↑ Ocular Pathology Atlas. American Academy of Ophthalmology Web site. https://www.aao.org/resident-course/pathology-atlas. Published 2016. Accessed January 4, 2017.
- ↑ Diabetes Control and Complications Trial Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology. 1995 Apr;102(4):647-61. doi: 10.1016/s0161-6420(95)30973-6. PMID: 7724182.
- ↑ 14.0 14.1 Ritch, R., Shields MB, Krupin T, The Glaucomas, St. Louis, MO: Mosby; 1989:1067.
- ↑ Chandler PA, and Grant, WM: Lectures on glaucoma, Philadelphia, 1965, Lea and Febiger.
- ↑ 16.0 16.1 Morrison, JC and Pollack IP, Glaucoma: Science and Practice, New York, NY: Thieme; 2003:226-236.
- ↑ 17.0 17.1 Schacknow P, Samples J. The Glaucoma Book: A Practical, Evidence-Based Approach to Patient Care. New York, NY: Springer; 2010:517-525.
- ↑ Mahdy RA, Nada WM, Fawzy KM, Alnashar HY, Almosalamy SM. Efficacy of intravitreal bevacizumab with panretinal photocoagulation followed by Ahmed valve implantation in neovascular glaucoma. J Glaucoma. 2013 Dec;22(9):768-72. doi: 10.1097/IJG.0b013e318259aec4. PMID: 22790513.
- ↑ Flanagan DW, Blach RK. Place of panretinal photocoagulation and trabeculectomy in the management of neovascular glaucoma. Br J Ophthalmol. 1983 Aug;67(8):526-8. doi: 10.1136/bjo.67.8.526. PMID: 6191770; PMCID: PMC1040112.
- ↑ Krupin T, Kaufman P, Mandell AI, Terry SA, Ritch R, Podos SM, Becker B. Long-term results of valve implants in filtering surgery for eyes with neovascular glaucoma. Am J Ophthalmol. 1983 Jun;95(6):775-82. doi: 10.1016/0002-9394(83)90064-8. PMID: 6190402.
- ↑ Molteno AC, Haddad PJ. The visual outcome in cases of neovascular glaucoma. Aust N Z J Ophthalmol. 1985 Nov;13(4):329-35. doi: 10.1111/j.1442-9071.1985.tb00443.x. PMID: 2421746.
- ↑ Ma KT, Yang JY, Kim JH, Kim NR, Hong S, Lee ES, Seong GJ, Kim CY. Surgical results of Ahmed valve implantation with intraoperative bevacizumab injection in patients with neovascular glaucoma. J Glaucoma. 2012 Jun-Jul;21(5):331-6. doi: 10.1097/IJG.0b013e31820e2fd0. PMID: 21673594.
- ↑ Chen CH, Lai IC, Wu PC, Chen YJ, Chen YH, Lee JJ, Liu YC, Kuo HK. Adjunctive intravitreal bevacizumab-combined trabeculectomy versus trabeculectomy alone in the treatment of neovascular glaucoma. J Ocul Pharmacol Ther. 2010 Feb;26(1):111-8. doi: 10.1089/jop.2009.0055. PMID: 20148654.
- ↑ Hampton C, Shields MB, Miller KN, Blasini M. Evaluation of a protocol for transscleral neodymium: YAG cyclophotocoagulation in one hundred patients. Ophthalmology. 1990 Jul;97(7):910-7. doi: 10.1016/s0161-6420(90)32482-x. PMID: 2381706.
- ↑ Callens C, D'Hondt K, Zeyen T. The long-term effect of diode laser cyclodestruction on intraocular pressure. Bull Soc Belge Ophtalmol. 2003;(289):81-6. PMID: 14619633.
- ↑ Hamard P, Gayraud JM, Kopel J, Valtot F, Quesnot S, Hamard H. Traitement des glaucomes réfractaires par cyclophotocoagulation transsclérale au laser semi-conducteur diode. Analyse de 50 patients suivis pendant 19 mois [Treatment of refractory glaucomas by transscleral cyclophotocoagulation using semiconductor diode laser. Analysis of 50 patients followed-up over 19 months]. J Fr Ophtalmol. 1997;20(2):125-33. French. PMID: 9099271.