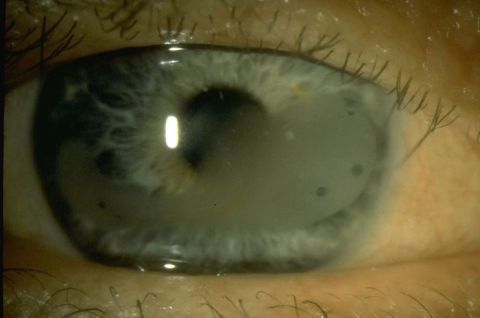
Calcific Band Keratopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Calcific Band Keratopathy (also known as Band Keratopathy). ICD 10: H18.429
Disease
Band keratopathy is a corneal degeneration that is most often composed of fine dust-like calcium deposits in the subepithelium, the Bowman layer, and the anterior stroma. It is typically a band-shaped, horizontal opacity that grows from the peripheral cornea toward the central cornea. Initial deposits are gray, but as the deposition progresses, the band becomes chalky-white. Band keratopathy can be related to systemic disease (autoimmune, renal disease), result from intraocular surgery or chronic intraocular conditions, or be idiopathic.[2]
Etiology
The etiology of band keratopathy can be idiopathic; however, systemic conditions are often an associated cause. A thorough history and medical workup can help identify the etiology of band keratopathy so that appropriate treatment of the underlying cause can be initiated.
Band keratopathy is caused by an imbalance of calcium and phosphate that initiates deposition into the cornea. This imbalance can be caused by systemic illnesses (such as renal disease, hyperparathyroidism, juvenile idiopathic arthritis, uveitis), intraocular/local processes, or alkalosis on the surface of the cornea, leading to decreased solubility of calcium. It can also result from keratoconjunctivitis sicca caused by increased evaporation of tears. The interpalpebral portion of the cornea has the most exposure to ambient air and leads to a high concentration and precipitation of calcium. Alkalosis also increases precipitation of calcium, resulting in corneal deposition, and is typically caused by chronic inflammation of the cornea. Therefore, any causes of uveitis can result in band keratopathy.[3][4][5]
In addition, endothelial compromise with associated corneal edema can also cause calcium deposition into the posterior cornea and result in band keratopathy. Aphakic patients who have a history of prolonged use of silicone oil in the eye have an 8% increased chance of that posterior corneal band keratopathy will develop, due to the contact of the oil with the posterior cornea. Intraocular tissue plasminogen activator can also cause band keratopathy. The association of these disease processes that leads to band keratopathy is currently unknown. Endothelial compromise may also cause band keratopathy to develop in ocular conditions such as end-stage glaucoma, corneal dystrophies, and neurotrophic keratitis.[3][6]
Topical medications may also be responsible for this condition. Ophthalmic drops containing phosphates or phosphate buffers such as steroids may be culprits. Similarly, mercury-based preservative drops such as pilocarpine initiates changes in the corneal collagen, increasing calcium deposition into that area. Occupations with exposure to mercury vapor or calcium bichromate vapors can cause corneal degeneration, leading to keratopathy. Finally, injury to the corneal surface, or surgeries that affect the corneal epithelium, also increase the likelihood that band keratopathy will develop. The extensive causes of band keratopathy make the patient history imperative to appropriate diagnosis and management.[3][4][5]
Summary of Etiology
- Chronic inflammatory ocular disease including uveitis (especially in association with juvenile idiopathic arthritis), chronic herpes simplex keratouveitis, glaucoma, and keratitis sicca, as well as exposure conditions including interstitial keratitis, climatic droplet keratopathy, phthisis bulbi, and corneal dystrophies
- Chemical exposure to mercury fumes, phosphate-containing drops (eg, certain steroid preparations, pilocarpine, older viscoelastic agents), intraocular silicone, thiazides, or calcium bichromate exposure
- Systemic hypercalcemia including hyperparathyroidism, vitamin D toxicity, sarcoidosis, milk-alkali syndrome, nephropathic cystinosis, hypophosphatemia, Paget disease, multiple myeloma, and cancer metastatic to bone
- Hereditary disorders including Norrie disease and congenital band keratopathy
- Other systemic diseases including discoid lupus, gout, tuberous sclerosis, ichthyosis
- Other causes including trauma to cornea, iris melanoma, and surgical interventions to the cornea (such as aphakia with silicone oil)[2][7]
Risk Factors
- Uveitis
- Keratitis sicca (evaporation of tears, leading to calcium-phosphate deposition on the interpalpebral zone)
- Trauma to cornea
- Surgery to cornea
- Systemic illnesses leading to hypercalcemia (eg, hyperparathyroidism, vitamin D toxicity, multiple myeloma, Paget disease of the bone – see etiology section for extensive list)
- Chemical or topical exposure (particularly mercury, phosphate, silicone oil, calcium bichromate, pilocarpine - see etiology section for extensive list)
- Alkalosis of cornea
- Retinopathy of prematurity [2][4][5][8]
General Pathology
The pathology for band keratopathy occurs from the calcium deposition (in hydroxyapatite form) into the Bowman layer, the epithelial basement membrane, and the anterior stroma. It initially presents as fine, microscopic particles into the Bowman layer, which deposit peripherally and extend centrally, ultimately resulting in a horizontal band-like plaque. As the calcific depositions continue, they coalesce to form denser plaques across the interpalpebral zone of the cornea, leading to significant visual impairment and discomfort.[2][3][9]
Pathophysiology
The pathophysiology of band keratopathy depends on the underlying cause that can lead to the development of corneal degeneration and calcium deposition. Any insult to the cornea, whether through systemic illness, chronic inflammation, direct or indirect injury, medications, or exposures, can lead to the development of the band keratopathy.
Band keratopathy is defined as a disease process that occurs from subepithelial calcium hydroxyapatite deposition leading to an opaque, band-like horizontal plaque across the cornea. This opacity often impacts the visual axis, leading to decreased visual acuity in patients. The calcium hydroxyapatite deposits are the most stable form of calcium and are found in human bone and teeth. As the disease progresses, the peripheral deposits of the band are gray, but they continue to progress centrally and develop a chalky-white color in a band-like distribution. Calcium deposits occur at sites of corneal injury or if there is a higher concentration of calcium on the ocular surface. This can occur due to systemic illness, hypercalcemia from secondary disease, alkalotic environment, decreased tears, or other exposures to the surface of the eye. As calcium hydroxyapatite becomes more insoluble on the ocular surface environment, it deposits into the epithelial basement membrane and anterior surface of the Bowman layer.
The distribution of the degeneration typically starts at the 3 o’clock and 9 o’clock peripheries of the cornea, eventually meeting centrally. The deposition of particles is slow and occurs over the course of months to years. There is also a very characteristic sharply demarcated lucent edge separating the limbus and the calcium deposition of the band keratopathy. This lucency may be due to the lack of Bowman layer in the periphery of the cornea or the increased buffering capacity of the limbal vessels that prevent the deposition of calcium. Lucent holes may also be visible within the band keratopathy, which represent corneal nerves penetrating through the Bowman layer.
The initial deposition of calcium starts in the epithelial basement membrane and the anterior surface of the Bowman layer. However, progression also leads to the deposit of the calcium into the anterior stroma, with an eventual break through the epithelium. With epithelial disruption, irritation, foreign-body sensation, and photophobia may occur. The calcium deposits can also extend posteriorly to penetrate the Bowman layer and enter the superficial stroma. The deposition most often occurs in the interpalpebral fissure because the highest exposure to ambient air occurs in that region; however, in severe cases, the keratopathy may progress inferiorly.
The condition can often be confused with climate droplet keratopathy and urate keratopathy. The distribution for all 3 disease processes is similar; however, the latter 2 lack calcium deposits. Characteristics of climate droplet keratopathy that distinguish it from band keratopathy include its occurrence in endemic regions with more globular deposits. Urate keratopathy is unique, as it has brown deposits.[2][3][7]
Diagnosis
Diagnosis of band keratopathy is primarily based on slit-lamp examination. The etiology of band keratopathy is extensive, so it is important to get a thorough history and serologic workup to identify any underlying causative pathology. In addition, slit-lamp examination will show the presence of a horizontal band across the cornea from 9 o’clock to 3 o’clock, which is pathognomonic for this disease.[2][7]
History
History is a key component of diagnosing band keratopathy. A thorough past medical history, employment history, previous ocular history and medication list should be acquired. Topical medications and the use of excessive vitamin D and calcium supplements may stimulate the insolubility of calcium hydroxyapatite, leading to corneal deposition.[2][7]
Physical Examination
Physical examination findings in band keratopathy include decreased visual acuity (often mild), with characteristic slit-lamp examination findings.:
- Gray-white plaque with fine, dusty deposits in a horizontal band distribution on the cornea
- Sparing of the extreme periphery of the cornea (normal lucency)
- Lucent holes in plaque (representing corneal nerves through Bowman membrane)
- Flakey, peripheral plaque[2][7]
Symptoms
- Decreased vision (usually mild)
- Foreign body sensation
- Ocular irritation
- Redness (occasionally)
- Photophobia
- Visible cosmetic changes to the eye[2][3]
Diagnostic Procedures
Baseline workup is recommended in patients with band keratopathy who do not have an obvious intraocular history (such as silicone oil placement, history of multiple surgeries), as a number of systemic illnesses may cause the disease to develop.[2][7] This includes:
- Urinalysis (deposits, pH)
- Serum calcium (high calcium leads to increased precipitate)
- Serum phosphorus (low phosphorus precipitates calcium, may indicate renal failure)
- Uric acid
- Blood urea nitrogen (BUN)
- Creatinine
- Parathyroid (PTH) levels
- Angiotensin-converting enzyme (ACE) and lysozyme levels (if sarcoidosis is suspected)
- Chest X-ray (if sarcoidosis is suspected)
Differential diagnosis
- Gout
- Interstitial keratitis
- Primary and secondary calcareous degeneration of the cornea
- Vitamin D deficiency
- Calciphylaxis
- Limbal stem cell deficiency
- Spheroidal degeneration
- Ciprofloxacin crystalline deposits (also ofloxacin and norfloxacin)
- Advanced basement membrane dystrophy or Salzmann nodular degeneration
- Familial band keratopathy [3]
Management
Medical therapy
Medical management should be the first-line treatment option in band keratopathy, especially in patients with past ocular surgery or underlying illness. Without treatment of the underlying cause, even with surgical management, the band keratopathy will most likely recur, or the cornea will have poor healing. Patients who have no symptoms or are mildly symptomatic are the optimal candidates for initiating medical therapy.
Medical therapy includes the use of a bandage contact lens with antimicrobial topical therapy to prevent the kind of infection that occurs with prolonged contact lens use. For patients concerned with the cosmetics of the eye, prosthetic opaque contact lenses can also be used. Artificial tears, gels, and ophthalmic ointments can help with irritation, tearing, foreign body sensation, and photophobia and is highly recommended in all patients with band keratopathy.
In addition, diet can play a role in the development of band keratopathy. Excessive vitamin D intake and milk-alkali syndrome can result in increased absorption and serum elevation of calcium, leading to band keratopathy. Baseline workup will show hypercalcemia, and appropriate dietary changes need to be placed.[2]
Surgery
Surgical management of band keratopathy is strongly considered after underlying systemic or ocular causes are treated or eliminated. Performing surgical treatment optimizes visual improvement. Almost 95% of patients had partial to complete symptomatic relief and almost two-line visual improvement. Various treatment options are available, and the most invasive treatments are reserved for extensive band keratopathy.[3]
Superficial debridement is the least invasive surgical management for band keratopathy, and it can be conducted in the clinic. To remove the deposits, the affected areas are scraped with a beaver blade and then treated with sponge or filter paper soaked in 3% ethylenediaminetetraacetic acid (EDTA). EDTA application occurs every 3 minutes until satisfactory removal of calcium deposits. EDTA can be toxic to the corneal surface; therefore, utilizing a soaked method and performing thorough irrigation after the procedure limits the contact of EDTA with the cornea and prevents toxicity. If removal of the deposits requires more extensive debridement, it can be completed in the minor-procedure room under topical anesthetics.[3][9]
It is significant to mention that despite the necessity of EDTA in adequate treatment of band keratopathy, it is difficult to obtain. EDTA can be made in compounding pharmacies. In a study by Lee and colleagues,[10] dipotassium-EDTA (K2-EDTA) was found to be an effective treatment for band keratopathy. K2-EDTA is found in blood draw tubes, and rinsing these tubes with a balanced salt solution and collecting the fluid was found to be an effective alternative treatment. K2-EDTA is relatively abundant in health care, more accessible, and a cost-effective alternate chelating agent for calcium band keratopathy.[9] Tripotassium ethylenediaminetetraacetic acid (K3-EDTA) at a concentration of 36 mg/mL has also been found to be effective. [11]
After adequate removal of the deposits, the cornea can be assessed for smoothness. Irregular surface post-debridement must be smoothed using a diamond burr or excimer laser phototherapeutic keratectomy. The popularity of excimer laser phototherapeutic keratectomy is due to its increased precision in removing the desired depth of anterior stroma, compared to manual superficial keratectomy. This precision allows for fewer post-procedure complications and decreased corneal scarring. Scanning excimer laser models have the same precision as the excimer laser phototherapeutic keratectomy, with decreased hyperopic shifts. However, excimer laser phototherapeutic keratectomy should not be used for calcium removal, because ablation of calcium is less effective than normal tissue. Uneven removal of the deposits can lead to irregular astigmatism, despite the polishing capacities of the lasers. In addition, excimer laser phototherapeutic keratectomy should be avoided in paracentral band keratopathy or band keratopathy with patchy visual axis involvement, because this can cause undesirable hyperopic refractive changes.[3][9][12]
Patients with chronic ocular surface inflammation, extensive band keratopathy, or advanced age may need to be treated by a specialist due to their increased risk of a nonhealing epithelial defects. Advanced cases, especially if calcium is deposited into the Bowman layer, may require a lamellar keratectomy technique with retrobulbar anesthesia. This is performed in the operating room with more extensive polishing to minimize irregular astigmatism. In extensive keratectomies, limbal bleeding may occur, which should be controlled with pressure or topical vasoconstrictors.[3]
Surgical follow-up
Postoperative care includes the therapeutic use of a bandage soft contact lens, which allows a framework for limbal stem cells to regenerate the cornea, protects from disruption of re-epithelialization of the cornea due to blinking, and gives significant pain relief. It is typically left in place until the corneal epithelium heals with use of topical nonsteroidal drops for pain control and antibiotic drops to prevent infection. It is imperative to avoid phosphate-containing topical agents, as it can precipitate calcium and cause a recurrence of the band keratopathy. Removal of the bandage soft contact lenses typically occurs within the first 2-weeks post procedure. Follow-up should occur every 3 to 12 months, depending on the recovery and postoperative symptoms.[3]
Complications
Complications post treatment of band keratopathy include: [2]
- Pain
- Recurrence of the calcium band
- Corneal scarring
- Corneal edema
- Infection
- Decreased vision or vision loss
- Persistent or aggravated irregular astigmatism
- Limbal stem cell deficiency
For residual corneal scarring, excimer laser phototherapeutic keratectomy can be considered for further improvement of vision.[3]
Prognosis
The prognosis of band keratopathy is generally favorable. It is imperative to treat underlying ocular or systemic conditions along with the band keratopathy, to have the lowest rate of recurrence.
Treatment with EDTA-chelation has the most significant improvement of vision and associated symptoms. Over 95% of patients have partial to complete symptomatic relief. However, if the visual axis is impacted or deposition reaches the posterior segment, visual improvement will be limited.[3][7][9]
References
- ↑ American Academy of Ophthalmology. Calcific Band Keratopathy https://www.aao.org/image/calcific-band-keratopathy-3 Accessed November 21, 2019.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 Calcific band keratopathy. American Academy of Ophthalmology. Basic and Clinical Science Course, Section 8. External Disease and Cornea. San Francisco: American Academy of Ophthalmology; 2025-2026:141-145.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Stokkermans TJ, Gupta PC, Sayegh RR. A Hands-on Approach to Band Keratopathy. Review of Optometry. https://www.reviewofoptometry.com/article/a-handson-approach-to-band-keratopathy. Published January 15, 2017. Accessed August 22, 2019.
- ↑ 4.0 4.1 4.2 Bernauer W, Thiel MA, Kurrer M, Heiligenhaus A, Rentsch KM, Schmitt A, Heinz C, Yanar A. Corneal calcification following intensified treatment with sodium hyaluronate artificial tears. Br J Ophthalmol. 2006 Mar;90(3):285-8. doi: 10.1136/bjo.2005.082792. PMID: 16488945; PMCID: PMC1856937. https://bjo.bmj.com/content/90/3/285.citation-tools. Accessed November 3, 2025.
- ↑ 5.0 5.1 5.2 Doostdar N, Manrique CJ, Hamill MB, Barron AR. Synthesis of calcium-silica composites: a route toward an in vitro model system for calcific band keratopathy precipitates. J Biomed Mater Res A. 2011 Nov;99(2):173-83. doi: 10.1002/jbm.a.33172. Epub 2011 Aug 16. PMID: 21976442.https://www.ncbi.nlm.nih.gov/pubmed/21976442. Published November 2011. Accessed November 3, 2025.
- ↑ Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology. 1988 Jul;95(7):870-6. doi: 10.1016/s0161-6420(88)33080-0. PMID: 3174036. https://www.ncbi.nlm.nih.gov/pubmed/3174036. Published July 1988. Accessed November 3, 2025.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 6 Donaghy C, Vislisel J, Greiner M, Goins K, Wagoner M. Calcific Band Keratopathy. EyeRounds.org. https://webeye.ophth.uiowa.edu/eyeforum/cases/214-band-keratopathy.htm. Published June 2, 2015. Accessed November 3, 2025.
- ↑ Hildebrand JC, Kerr NC. Band Keratopathy in treated Retinopathy of Prematurity. Paper presented at: 45th Annual Meeting of American Association for Pediatric Ophthalmology and Strabismus; March 27, 2019; San Diego, CA.
- ↑ 9.0 9.1 9.2 9.3 9.4 Najjar DM, Cohen EJ, Rapuano CJ, Laibson PR. EDTA chelation for calcific band keratopathy: results and long-term follow-up. Am J Ophthalmol. 2004 Jun;137(6):1056-64. doi: 10.1016/j.ajo.2004.01.036. PMID: 15183790.
- ↑ Lee M, Ouano D, Shapiro B, Fong A, Minas Coroneo. "Off-the-Shelf" K2-EDTA for Calcific Band Keratopathy. Cornea. https://insights.ovid.com/pubmed?pmid=29489518. Published July 1, 2018. Accessed November 3, 2025.
- ↑ Guo Z, Henry RK, Dastjerdi MH. Comparative Analysis of Alternative Calcium Chelators for the Treatment of Calcific Band Keratopathy. Cornea. 2023 Dec 1;42(12):1551-1554. doi: 10.1097/ICO.0000000000003370. Epub 2023 Aug 21. PMID: 37603695.
- ↑ Garg S, McColgin AZ, Steinert RF. Phototherapeutic Keratectomy. American Academy of Ophthalmology. https://www.aao.org/munnerlyn-laser-surgery-center/phototherapeutic-keratectomy-3. Published November 12, 2013. Accessed Novemmber 3, 2025.