Faricimab
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Overview
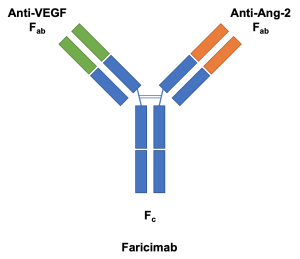
Faricimab-svoa (Vabysmo™, Genentech, San Francisco, CA) is a combined-mechanism medication with simultaneous and independent binding[1] on both vascular endothelial growth factor A (VEGF-A) and angiopoietin-2 (Ang-2) that is approved for the treatment of diabetic macular edema and neovascular (wet) age-related macular degeneration (nAMD) by the FDA on January 2022 (Food and Drug Administration, USA).
Indications and Use
Faricimab has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of
- diabetic macular edema,
- wet age-related macular degeneration, and
- macular edema secondary to retinal venous occlusion (added on 26 October 2023).
Mechanism of Action
Faricimab has an inhibitory effect on both VEGF-A and Ang-2 and is thought to have a longer-lasting effect than previous anti-VEGF agents in clinical trials.
Anti-VEGF therapy has been employed in the treatment of retinal vascular disease, however, its effectiveness is limited by the role of VEGF in pathogenesis.[2]
The anti-VEGF-A effect
- inhibits endothelial proliferation,
- reduces vascular permeability, and
- suppresses neovascularization.
Exploration of alternative targets has revealed the role of angiopoietin (Ang), whose two isoforms, Ang-1 and Ang-2, bind to tyrosine kinase (Tie-2) endothelial receptors to regulate vasculogenesis.[2] Ang-1 is an agonist of Tie-2 with constitutive vessel-stabilizing effects,[3] but Ang-2 is an antagonist which inhibits its phosphorylation and has been shown to play a role in cytokine-induced vascular leakage.[3] Ang-2-deficient (Ang-2-/-) mice even demonstrate a diminished vascular response to VEGF.[3]
The anti-Ang 2 effect is thought to
- improve vascular stability and
- desensitize the vessels to the actions of VEGF-A.
The use of monoclonal antibodies in anti-VEGF therapy presented an opportunity for targeting two mediators of retinal vascular disease with a single molecule.[2] These bispecific antibodies may be developed with CrossMAb (Roche, Basel, Switzerland), a proprietary technology that allows for two different antigen-binding domains (Fab) on the antibody.[4][5] CrossMAb was used in the development of faricimab, originally known as RG7716.[2]
Administration and Dosing
Faricimab is given as an intravitreal injection at a dose of 6 mg from 0.05 mL of a 120 mg/mL solution.[6] For neovascular age-related macular degeneration, one regimen is 6 mg intravitreal every 4 weeks for the first 4 doses, then the same dose either 8 or 12 weeks later depending on findings of optical coherence tomography and visual acuity evaluations. [1]
According to the FDA label: 'The recommended dose for VABYSMO is 6 mg (0.05 mL of 120 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 ± 7 days, monthly) for the first 4 doses, followed by optical coherence tomography and visual acuity evaluations 8 and 12 weeks later to inform whether to give a 6 mg dose via intravitreal injection on one of the following three regimens: 1) Weeks 28 and 44; 2) Weeks 24, 36 and 48; or 3) Weeks 20, 28, 36 and 44. Although additional efficacy was not demonstrated in most patients when VABYSMO was dosed every 4 weeks compared to every 8 weeks, some patients may need every 4-week (monthly) dosing after the first 4 doses. Patients should be assessed regularly.'
For diabetic macular edema (DME), faricimab is injected every 4 weeks for the first 4 doses. Then, a personalized treatment interval similar to treat and extend may be implemented. The dosing interval may then be adjusted based on the response of macular edema as measured by optical coherence tomography.[6] Alternatively, a 6 mg intravitreal injection can be administered every 4 weeks for the first 6 doses, followed by an injection every 8 weeks over the next 28 weeks.[6]
The details of the dosage in DME are mentioned in FDA label as: 'VABYSMO is recommended to be dosed by following one of these two dose regimens: 1) 6 mg (0.05 mL of 120 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 days ± 7 days, monthly) for at least 4 doses. If after at least 4 doses, resolution of edema based on the central subfield thickness (CST) of the macula as measured by optical coherence tomography is achieved, then the interval of dosing may be modified by extensions of up to 4-week interval increments or reductions of up to 8-week interval increments based on CST and visual acuity evaluations through week 52; or 2) 6 mg dose of VABYSMO can be administered every 4 weeks for the first 6 doses, followed by 6 mg dose via intravitreal injection at intervals of every 8 weeks (2 months) over the next 28 weeks. Although additional efficacy was not demonstrated in most patients when VABYSMO was dosed every 4 weeks compared to every 8 weeks, some patients may need every 4-week (monthly) dosing after the first 4 doses. Patients should be assessed regularly.'
For macular edema following retinal venous occlusion: 'The recommended dose for VABYSMO is 6 mg (0.05 mL of 120 mg/mL solution) administered by intravitreal injection every 4 weeks (approximately every 28 ± 7 days, monthly) for 6 months.'
Preparation
Faricimab is a 120 mg/mL solution in a single-dose vial that should be refrigerated prior to use.[6] Each vial contains more solution than necessary for the administration of a single 0.05 mL dose of the solution containing 6 mg of faricimab.[6] Before injection, the eye should be anesthetized with a topical anesthetic and cleansed with betadine. The contents of the faricimab vial should be drawn using the provided 18 gauge needle. The 18 gauge needle should then be replaced with a sterile 30 gauge ½-inch needle for the injection.[6]
Clinical Trials
Faricimab has been the subject of multiple clinical trials to evaluate its safety and efficacy.
BOULEVARD and STAIRWAY are phase II clinical trials of faricimab for diabetic macular edema (DME) and neovascular age-related macular degeneration (nAMD), respectively.[2][1] RHINE and YOSEMITE are phase III trials of faricimab in DME.[2] TENAYA and LUCERNE are phase III trials of faricimab in nAMD.[2][7]
BOULEVARD
BOULEVARD is a phase II, 36-week, double-blind, randomized, active comparator-controlled, multicenter trial of 229 treatment-naïve patients with DME.[2][8] Patients were randomized into one of three cohorts: 6.0 mg faricimab, 1.5 mg faricimab, or 0.3 mg ranibizumab. Patients were treated Q4W for 20 weeks. Primary endpoint outcomes were measured at 24 weeks and followed until 36 weeks.[2] The 6.0 mg faricimab cohort demonstrated superior letter gains in visual acuity, central subfield thickness reduction, and diabetic retinopathy severity score (DRSS) improvement. The findings suggested a benefit in visual gain and potential for extended durability[9] of simultaneous inhibition of Ang-2 and VEGF-A in patients with DME.[8]
YOSEMITE and RHINE
YOSEMITE and RHINE are identically designed phase III clinical trials of faricimab in treatment-naive and previously anti-VEGF-treated patients with center-involving diabetic macular edema.[9] The studies compared faricimab Q8W after 6 initial Q4W doses, faricimab personalized treatment interval (PTI) after 4 initial Q4W doses, and aflibercept Q8W after 5 initial Q4W doses.[9] PTI is based on treat-and-extend and uses prespecified best-corrected visual acuity (BCVA) and central subfield thickness (CST) criteria.[9] The primary efficacy endpoint is the mean change in BCVA from baseline averaged over weeks 48, 52, and 56. One-year results were presented at ARVO 2021.[9]
TENAYA and LUCERNE
TENAYA and LUCERNE are identical, phase III, randomized, double-masked, active comparator-controlled, 112-week studies of faricimab in neovascular AMD.[7] Patients were randomized to receive either faricimab or aflibercept during the study period.[7] The primary efficacy endpoint was mean change in best-corrected visual acuity (BCVA) from baseline averaged over weeks 40, 44, and 48.[7] Secondary endpoints included the proportion of patients on faricimab Q16W, Q12W, and Q8W regimens, proportion of patients gaining ≥15 or ≥10 ETDRS letters from baseline, and changes from baseline in anatomic outcomes.[7] Safety outcomes included incidence and severity of ocular and nonocular adverse events.[7] One-year results were presented at ARVO 2021.[7]
STAIRWAY
STAIRWAY is a phase II, 52-week randomized, active comparator-controlled, parallel-group, multicenter study that evaluated 76 patients with nAMD on Q12W or Q16W dosing schedules after a loading dose of four monthly injections of faricimab compared to ranibizumab 0.5 mg Q4W.[1] Faricimab administered Q12W or Q16W after a loading dose demonstrated visual and anatomic improvement comparable to Q4W ranibizumab.[1]
BALATON and COMINO
BALATON and COMINO study evaluated macular edema in branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO) or hemiretinal venous occlusion respectively. These multicenter, phase 3, double-masked randomized control trials showed noninferiority of faricimab to aflibercept in treating these conditions.[10] The patients were 1:1 randomized to intravitreal aflibercept 2 mg or intravitreal faricimab 6 mg every 4 weeks till 20 weeks. Then, a treat and extend regimen of faricimab 6 mg up to every 4 months was used.
Other studies on faricimab
AVONELLE-X: to study long-term safety and tolerability of faricimab in nAMD as an extension study of TENAYA and LUCERNE
RHONE-X: to study long-term safety and tolerability of faricimab in DME as an extension study of YOSEMITE and RHINE
ELEVATUM: phase 4 study of faricimab in underrepresented patient populations (including black and Hispanic patients) with DME,
SALWEEN: to study faricimab in polypoidal choroidal vasculopathy
VOYAGER: to collect real-world long-term data on the use of faricimab in DME globally
Adverse reactions
Like all intravitreal anti-VEGF injections, faricimab injection has multiple potential risks, including subconjunctival hemorrhage, hypersensitivity, increased intraocular pressure, endophthalmitis, retinal detachment, vitreous hemorrhage, and thromboembolic events. The potential immunogenicity of the drug may lead to the development of anti-faricimab antibody in around 8-10% of cases.[11] Well-controlled studies are lacking in pregnant women regarding the use of this drug. The effect of this drug on the reproductive capacity of humans is unknown, and effective contraception is advised in women of reproductive potential who receive this drug. The contraception should be continued at least 3 months after the last dose of faricimab.[11] Retinal vasculitis and vascular occlusions can occur in around 0.06 per 10,000 injections.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Khanani AM, Patel SS, Ferrone PJ, et al. Efficacy of Every Four Monthly and Quarterly Dosing of Faricimab vs Ranibizumab in Neovascular Age-Related Macular Degeneration: The STAIRWAY Phase 2 Randomized Clinical Trial. JAMA Ophthalmol. 2020;138(9):964–972. doi:10.1001/jamaophthalmol.2020.2699
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Sharma A, Kumar N, Kuppermann BD, Bandello F, Loewenstein A. Faricimab: expanding horizon beyond VEGF. Eye (Lond). 2020;34(5):802-804. doi:10.1038/s41433-019-0670-1
- ↑ 3.0 3.1 3.2 Benest AV, Kruse K, Savant S, Thomas M, Laib AM, Loos EK, Fiedler U, Augustin HG. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One. 2013 Aug 5;8(8):e70459. doi: 10.1371/journal.pone.0070459. PMID: 23940579; PMCID: PMC3734283.
- ↑ Schaefer W, Regula JT, Bahner M, Schanzer J, Croasdale R, Durr H, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci USA. 2011;108:11187–92. doi: 10.1073/pnas.1019002108.
- ↑ Regula JT, Lundh von Leithner P, Foxton R, et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases [published correction appears in EMBO Mol Med. 2019 May;11(5):]. EMBO Mol Med. 2016;8(11):1265-1288. Published 2016 Nov 2. doi:10.15252/emmm.201505889
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Vabysmo prescribing information - gene.com. https://www.gene.com/download/pdf/vabysmo_prescribing.pdf. Accessed April 17, 2022
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Khanani AM, Heier J, Ruiz CQ, et al. Faricimab in neovascular age-related macular degeneration: 1-year efficacy, safety, and durability in the phase 3 Tenaya and Lucerne Trials. Investigative Ophthalmology & Visual Science. https://iovs.arvojournals.org/article.aspx?articleid=2772827. Published June 21, 2021. Accessed May 29, 2022.
- ↑ 8.0 8.1 Sahni J, Patel SS, Dugel PU, et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology. 2019;126(8):1155-1170. doi:10.1016/j.ophtha.2019.03.023
- ↑ 9.0 9.1 9.2 9.3 9.4 Wells JA, Wykoff CC, Willis JR, et al. Efficacy, durability, and safety of faricimab in diabetic macular edema (DME): one-year results from the phase 3 YOSEMITE and RHINE trials. Investigative Ophthalmology & Visual Science. 2021;62(8):1037.
- ↑ Tadayoni R, Paris LP, Danzig CJ, Abreu F, Khanani AM, Brittain C, Lai TYY, Haskova Z, Sakamoto T, Kotecha A, Schlottmann PG, Liu Y, Seres A, Retiere AC, Willis JR, Yoon YH; BALATON and COMINO Investigators. Efficacy and Safety of Faricimab for Macular Edema due to Retinal Vein Occlusion: 24-Week Results from the BALATON and COMINO Trials. Ophthalmology. 2024 Jan 26:S0161-6420(24)00090-3. doi: 10.1016/j.ophtha.2024.01.029. Epub ahead of print. PMID: 38280653.
- ↑ 11.0 11.1 DailyMed - VABYSMO- faricimab injection, solution [Internet]. [cited 2022 Aug 31];Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=04cc9ef7-c02a-4e92-a655-0062674e8487