Ab Interno Trabeculectomy and Trabeculotomy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Glaucoma is one of the most prevalent causes of blindness in the world and can be treated by controlling elevated intraocular pressure (IOP) in patients. If left untreated, glaucoma can progress from peripheral blindness to complete blindness. There are many forms of glaucoma with primary open-angle glaucoma being the most common. Management usually begins medically with topical pressure-lowering drops first until max medical therapy is reached--usually 4 different classes of IOP-lowering drops as well as an oral carbonic anhydrase inhibitor. Treatment after that depends on the mechanism of the underlying glaucoma, but generally involves laser therapy or various types of surgeries.[1]
An increasingly popular treatment option is a group of surgeries called minimally or microinvasive glaucoma surgery (MIGS), which have shown to have minimal risk with a mild compromise in efficacy. The most attractive features of these surgeries and largely why they are often a primary surgical option are their incredibly high safety profile and very low rates of serious complications.[2] Several various types of MIGS procedures have evolved over time including Schlemm’s canal (SC) devices, subconjunctival devices, and the method this article will exploring, ab interno trabeculectomy and ab interno trabeculotomy (collectively referred to AIT).[3]
The ab interno approach aims to decrease IOP by increasing aqueous outflow through a direct opening in the trabecular meshwork (TM) from within the anterior chamber (AC) to produce direct communication from the AC to the outer wall of SC and the collector channels. This is in contrast to ab externo procedures which create that connection from the conjunctival side, requiring conjunctival dissection and does not involve significant manipulation within the AC. This article will focus on AIT, which includes the Trabectome (NeoMedix Corporation, Tustin, CA), Kahook Dual Blade (KDB, New World Medical, Rancho Cucamonga, CA) goniotomy, gonioscopy assisted transluminal trabeculotomy (GATT), and Omni (Sight Sciences, Menlo Park, CA). For more information about ab interno and ab externo canaloplasty, please visit the Canaloplasty page.
Surgery
Trabectome
The Trabectome is an ab interno trabeculectomy device that can be used to ablate the TM. The device was invented by George Baerveldt with the associated patent filed in 2002.[4] The commercial device is manufactured by the NeoMedix Corporation (Tustin, CA) and is marketed for the “microsurgical management of adult and infantile glaucoma”. The device was cleared by the United States Food and Drug Administration (FDA) in April 2004. Initial cases were performed in Mexico, with the first procedures conducted in the US occurring in January 2006 for the treatment of open-angle glaucoma.[5]
The Trabectome instrument utilizes a bipolar 550 kHz electrode, with adjustable power, to ablate the TM.[6] Unlike in cautery, plasma generated here has a highly confined heat dissipation cone with minimal thermal transfer to the outer wall (about 1.2C). The device is equipped with a footplate that helps guide the electrosurgical tip and protect adjacent tissues. Constant infusion further helps to maintain the chamber and tissue debris is removed by aspiration through the shaft of the device.[5]
Key Steps in Trabectome Surgery
If the case requires both Trabectome and phacoemulsification, the Trabectome portion should be conducted first.
The eye is pressurized with intracameral lidocaine or BSS through a paracentesis and the 1.6 mm iris-parallel clear-cornea and uniplanar main incision is made 2 mm anterior to the limbus to prevent iris prolapse. The inner third of the incision is flared to improve mobility of the handpiece and visualization by eliminating corneal striae from torque. In order to deepen the angle and enhance viewing, the irrigation bottle should be increased as high as determined to be safe for the stage of glaucoma with continuous irrigation. Viscoelastic should not be used to avoid optical interfaces and the trapping of ablation gas bubbles. In order to aid in position of the patient, the microscope tilt can be increased or the patient’s axial core rotated if they have significant difficulty with rotating their neck.[6]
After insertion of the Trabectome tip into the incision, the TM is engaged with the instrument at a 45˚ upward angle at the level of the scleral spur. It is more straightforward to begin ablation to the left of the main wound instead of directly opposite it (in a right-handed surgeon), as this allows a greater acute angle of contact between the Trabectome tip and the TM. When properly inserted, the TM should enter between the electrode tip and the footplate, causing the footplate to be obscured by the translucent TM. The leading, round, golden electrode should not directly press forward against the TM in ablation direction.
During the procedure, it is crucial to eliminate any outward push on the SC as this can cause damage to the posterior wall of SC and collector channel orifices. It is therefore important to apply a slight inward pull during ablation to offset any tendency to push or rub outward against the SC with unnecessary force.[6] Ablation should commence at an energy input of 0.8 mW and be increased as necessary. Coagulation necrosis can result with energies in excess of 1 mW, which manifest as darkening along the edge of the ablated tissue. This should be strictly avoided as it can initiate wound healing and associated complications including failure. Ablation of the first 60˚ can be achieved continuously without adjustments and an additional 30˚ can be completed by rotating the gonioscopy lens in the direction of the ablation while increasingly supinating the wrist. The tip is then disengaged and inverted downward away from the cornea so that ablation can proceed at the original starting point in the opposite direction. The target is to ablate 90˚ in each direction for a total of 180˚ per incision. Following a successful procedure, the outer wall of the SC will appear white.
After completion of electrosurgery, the anterior chamber can be injected with a small amount of viscoelastic material to protect the ablation arc and minimize postoperative hyphema from flow back.[6]
Kahook Dual Blade (KDB) Goniotomy
The Kahook Dual Blade (KDB, New World Medical, Rancho Cucamonga, CA) is a novel goniotomy blade created to produce a more complete removal of TM through a minimally invasive approach. It was launched in the United States in 2015 and was designed to achieve near complete removal of TM with minimal, if any, surrounding tissue damage. This is in contrast to other ab interno trabeculectomy techniques (Gonioscopy-Assisted Transluminal Trabeculotomy (GATT) and traditional trabeculotomy), where the TM is incised and therefore residual TM leaflets remain post-procedure. In addition, the Trabectome (Neomedix Corporation, Tustin, CA) has been shown to both have residual leaflets and cause thermal injury to nearby tissues. [7]A pre-clinical study on human donor corneo-scleral rims demonstrated histologically the more complete TM removal with less surrounding tissue damage with the KDB compared to both Trabectome and an MVR blade trabeculotomy.[7] [8]Minimizing residual trabecular meshwork leaflets theoretically may lead to less fibrosis overtime and therefore potentially enhance long term outcomes compared to other similar procedures.
The dual blade has several key design features to achieve a more complete goniotomy. First is the sharp tip designed with a taper to allow for smooth entry of the blade through TM and into Schelmm’s Canal (SC). The heel of the device fits easily within SC and allows smooth advancement of the blade within the canal while preventing collateral damage during treatment. The ramp of the blade generates a gentle stretch of the TM as the blade is advanced. Finally, the dual blades create parallel incisions of the TM allowing excision of a strip of TM which achieves a near complete removal of TM, instead of incision only.[7][8] In a clinical trial, the Kahook Dual Blade was found to excise a greater cross-sectional area of tissue compared to standard trabeculotomy.[9]
An additional benefit of the KDB, is that it is a single use, disposable instrument which does not require any additional special surgical equipment. Further, there is no implant related risks as no implant is left behind with this procedure.
The Kahook Dual Blade goniotomy is most commonly performed under topical anesthesia. In children or less cooperative adults, it can be performed under general anesthesia. For patients that require general anesthesia, it is important to remind the anesthesia team that rotation of the patient’s head will be required before performing such maneuvers. Preoperative pilocarpine and/or intracameral miotic agents can be used in patients undergoing the procedure without phacoemulsification to facilitate visualization of the angle, but is not required.
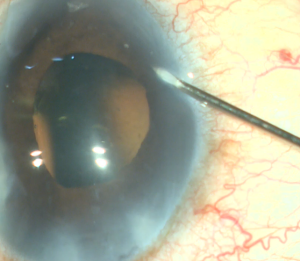
A corneal paracentesis is first created and the anterior chamber and angle are deepened with viscoelastic. Non-preserved lidocaine may be instilled intracamerally before viscoelastic for further anesthesia. A temporal clear corneal incision of at least 1.5mm is then created. More viscoelastic is added if necessary to deepen the nasal angle. Care should be taken not to over-inflate the eye as this can cause collapse of and difficulty entering the canal. Likewise, under inflation of the anterior chamber may result in corneal striae with gonioscopy. Next, the patient’s head is tilted approximately 30-45 degrees away from the surgeon and the microscope is tilted 45 degrees towards the surgeon. Viscoelastic is placed on the underside of a direct gonioprism and the device is inserted through the corneal incision sideways with the surgeon’s dominant hand while the gonioprism is placed on the ocular surface with the non-dominant hand so that the nasal TM is in direct view. If anatomic structures are difficult to identify, Trypan blue can be used to stain the TM, or the IOP can be lowered temporarily to produce blood reflux into SC. The sharp tip of the blade is inserted through the TM and into SC. After the TM is pierced, the heel of the device is seated against the wall of SC and advanced in a clockwise or counterclockwise manner for approximately 3-5 clock hours depending on surgeon preference. As the device is advanced, the ramp gently stretches the TM while the dual blades create parallel incisions to generate a strip of TM.[7] [8] If a large trabecular strip is present this can be removed with intraocular forceps. Small and/or peripheral strips however can be left in place. Viscoelastic is then irrigated from the anterior chamber and Carbachol intraocular solution may be injected intracamerally to produce pupillary miosis. All wounds are hydrated and checked to ensure water-tight closure and a 10-0 nylon is placed in the main wound if necessary. It is recommended that IOP be kept slightly high, around 20-25 mm Hg, at the end of the case to prevent further blood reflux, although this is patient and surgeon dependent.
When performed along with cataract surgery the goniotomy can be performed either before or after phacoemulsification at the surgeons’ preference as one study showed no difference in visualization as rated by surgeons whether the procedure was performed before or after phacoemulsification.[10] When surveyed, 98% of surgeons agreed that the use of the blade was straightforward and 98-99% agreed the advancement of the blade within SC was efficient.[11][12]
Gonioscopy-Assisted Transluminal Trabeculotomy (GATT)
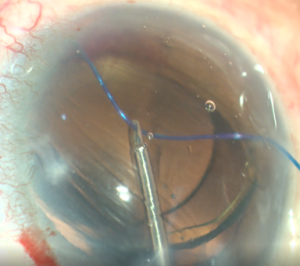
GATT is an ab interno trabeculotomy device that begins with entering the AC through a temporal corneal incision. A goniotomy is created in the nasal angle. A microcatheter or suture is then placed into SC and advanced circumferentially using microsurgical forceps.[8] Traction on the inserted microcatheter or suture creates a full 360° trabeculotomy, which does not involve removing any TM.[8][13]
The idea of using a Nylon suture to perform a trabeculotomy was first described by Smith et al. in 1962[14]. In 2012, Chin et al. published a study in Japan using the Nylon suture to perform a 360° trabeculotomy.[15] Later in 2014, Fellman and Grover described GATT using a Prolene suture[13], using large cohorts afterward to support its value.[16] [17] Since then, GATT surgery has been increasingly performed. Techniques such as AbiC, OMNI, and iTrack were later developed.
Before starting, one should ensure that the equipment, material, staff, security checklist, and positioning of the patient are done (a reverse Trendelenberg position is recommended to decrease blood reflux and improve the view). A temporal approach for the surgeon is preferred by the authors, but a superior position can also be performed. Then, we will perform a 180° GATT procedure.
Procedure
The video below shows the steps needed for the GATT surgery.
- A Paracentesis is created if combined phacoemulsification is planned. All incisions are corneal and not limbal to avoid bleeding from the limbal vessels that would hinder visualization. Next, intracameral lidocaine is injected, followed by viscoelastic in the anterior chamber (AC) and in the angle.
- With a 2.2 mm knife, create the main wound. If a combined cataract procedure is planned, it is commonly performed prior to IOL insertion (some surgeons prefer performing GATT before phacoemulsification). A 27-gauge needle is inserted, directed to the nasal angle.
- Cut the needle from the polypropylene 5-0 suture and use 6 cm of it, and then use a low-temperature cautery applied 90 degrees from the polypropylene 5-0 to induce a mushroom head of the suture. Then, insert it into the AC using the needle entry.
- Use the angular position: Tilt the head of the patient away from yourself at least 30 degrees, tilt the microscope towards yourself ≈40 degrees, and ask the patient to look away as a last resort if unable to achieve proper positioning from the head and microscope positioning alone. Use a gonio prism (such as a Swan-Jacob lens) to see whether the angle visualization is good.
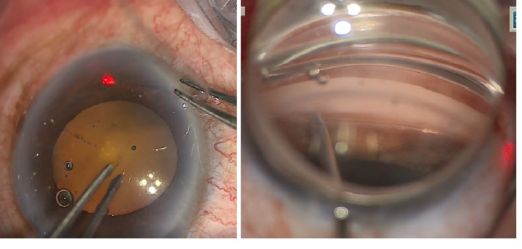
- An MVR blade or 27-gauge needle enters through the main wound and into the trabecular meshwork (TM) to perform a goniotomy (the blade is kept parallel to the TM or slightly anterior, starting to the right of the surgeon’s visual field and performing a goniotomy with one straight cut to the left of the surgical view and for of 1-clock-hour). A left-handed surgeon will start a goniotomy to the left of the view; the goniotomy should be on the side of the view that allows for maximum real estate to watch the suture go through Schlemm's canal, or to reserve space for a 2nd goniotomy if needed.
- Confirm the opening and extension of the goniotomy with gentle pressure posteriorly with the MVR blade.
- Viscoelastic (OVD) can be injected to dilate the Schlemm’s canal, which can decrease intraoperative bleeding and improve the view. Inject OVD to displace any bleeding away from the direction you will be inserting the suture (to the right for a right-handed surgeon).
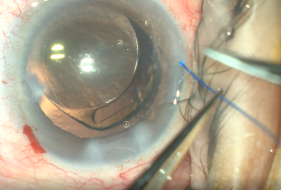
- Insertion of polypropylene suture into Shlemm’s canal. Using microsurgical iris forceps to hold the suture, insert the suture in the goniotomy and into Schlemm's canal. If the suture is in a good position (youcan appreciate the suture through the transparency of the Schlemm’s canal), perform 10 passes (or 20 small passes) to perform a trabecutolomy of 180 degrees. If the patient experiences any pain, stop to ensure the suture has not gone suprachoroidal, or that it has come out of Schlemm’s canal and on iris.
- Pull the suture centripetally to perform a trabeculotomy under a direct angular view. Then, after realigning the head of the patient and microscope, using the iris forceps again, pull the suture entirely into the center of AC and outside the eye . Finally, remove the OVD (leave up to 10%-40% of OVD in the anterior chamber to avoid postoperative bleeding, but care must be taken to prevent postoperative IOP pikes). The wounds should be thoroughly hydrated to ensure they are water-tight, and the eye should be left with an IOP >30 mm Hg to decrease the risk of bleeding.
The videos below show examples of GATT
Surgical Variations
- 180° versus 360° GATT Acumulating evidence suggests that 360° treatment of the Trabecular Meshwork (TM) or Schlemm’s canal (SC) may not be necessary to maximize the effect and achieve surgical success[18][19][20][21]. The most common hypotheses for this are a potential dose-response threshold effect beyond 120-180° of TM and non-uniform drainage along the 360° circumference of the TM, which results in increased efficacy with treatment to specific segments of TM[22] and less contribution to the effect with treating other areas (in healthy eyes a segmental active outflow occurs mainly in nasal and inferior quadrants)[23][24]. While success rates are similar with 360° and 180° trabeculotomy, the rates and severity of complications, particularly hyphema, may be higher with 360° cleavage of SC’s roof[18][24]. Therefore, according to the above-stated, the authors tend to perform hemi-GATT (unroofing of 180° of SC) procedures as a routine. Performing a 180°-GATT may also leave the remaining 180° for future intervention if needed.
- Inferior versus superior GATT Not much evidence exists comparing superior to inferior hemi-GATT procedures. Waldner et. Al. showed recurring trends of the lower hazard ratio for failure and better IOP reduction with performing inferior hemi-GATT rather than superior, though no statistical significant difference was found[25]. This is consistent with previous in-vitro and in-vivo anatomical studies, showing segmental outflow[22][26]. Further studies are required to assess this subject better. Likely there is variability in the distribution of collector channels between individuals. Ideally, one could determine whether an individual has superior or inferior dominance in their collector channel distribution, and subsequently, the hemi-GATT would be performed in the corresponding region (this point is still under discussion by glaucoma experts). We have described this variability by performing intraoperative venography, as seen in the videos below.
- Using devices for GATT GATT can be performed using devices such as iTrack and OMNI. OMNI allows the injection of viscoelastic as the catheter is retracted. iTrack also allows this, and it has a light that will enable visualization of the end of the cannula, allowing, for instance, detection and correction of a suprachoroidal or collector channel path. However, there is a cost for these devices.
- Endoscopic video assisted transluminal trabeculotomy (EATT) One can consider an endoscopic approach to GATT when the cornea precludes a gonioscopic view. Preoperatively a UBM and anterior segment OCT should be performed to assess the angle anatomy, and when there is a question of significant PAS that could not be released easily, one should discuss with the patient and be ready to consider a subconjunctival filtering surgery option if the EATT is not possible. For this technique, a separate incision is performed inferiorly for a right eye and right-handed surgeon, and superiorly for a left eye and right-handed surgeon (opposite for a left-handed surgeon). The microscope does not need to be tilted for this procedure, nor does the patient’s head need to be tilted. An endoscopic probe is inserted into the eye, and all the steps can be performed utilizing this endoscopic view.
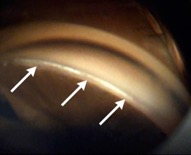
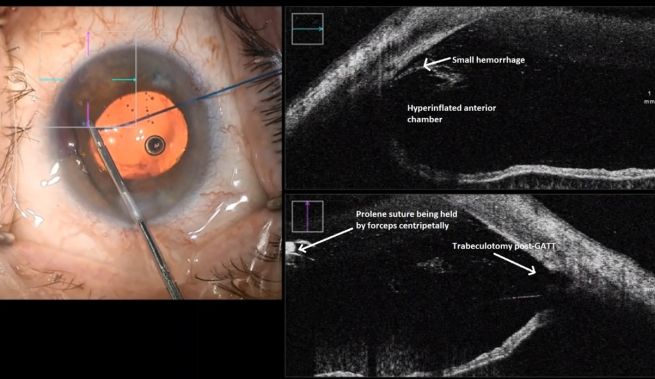
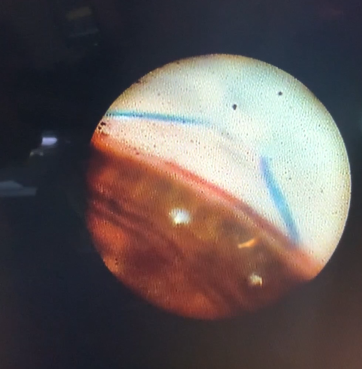 This endoscopic camera allows a visualization from the inside out; in the figure, there is a depiction of the final stage of the GATT: a trabeculotomy, with the suture being removed from the eye by the main incision (center of the image) and causing a trabeculotomy (right of the image)(Image courtesy of Matthew Schlenker, MD)
This endoscopic camera allows a visualization from the inside out; in the figure, there is a depiction of the final stage of the GATT: a trabeculotomy, with the suture being removed from the eye by the main incision (center of the image) and causing a trabeculotomy (right of the image)(Image courtesy of Matthew Schlenker, MD) - Using intraoperative OCT With the possibility of performing intraoperative OCT, we can have another means of verifying if the polypropylene 5-0 is in the proper position and see the trabeculotomy site.
Pearls for Success
The biggest pearls for success with this surgery are maintaining a good view and avoiding the suprachoroidal trajectory.
To ensure a good view, first, we must stop blood thinners and position the patient in reverse Trendlenburg, as placing the head above the heart diminishes episcleral venous pressure and blood reflux. We should be thorough in obtaining an excellent angular view (increasing the inclination of the head of the patient 30-45° away from the surgeon, the oculars of the microscope tilted closer to the surgeon at least 30°), and if needed asking the patient to look away from the surgeon; the gonioscopic lens should be held without excessive pressure to avoid corneal folds.distinguishes a top-down view from a good angular perspective. Don’t hesitate to use abundant OVD to expel bleeding and improve the surgical sight (ideally, from left to right of the field to direct the bleeding away from our surgical viewpoint for a right-handed surgeon).
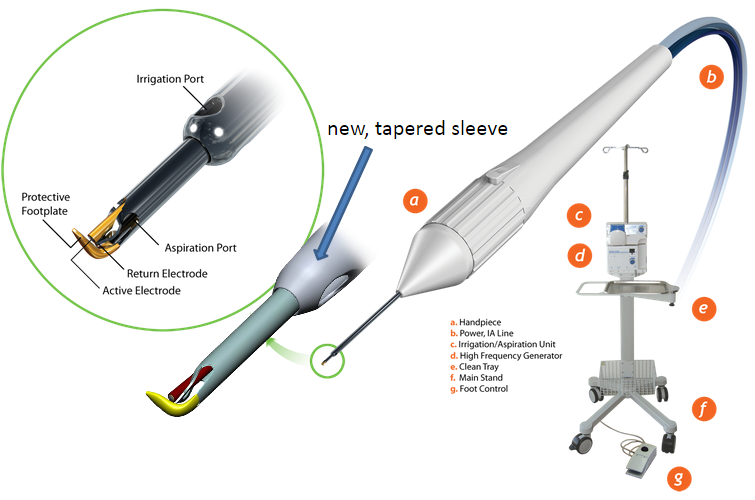
OMNI
Omni is also an ab interno trabeculotomy device, so in this sense, it is similar to GATT. During this procedure, the AC is similarly entered through a temporal corneal incision and a cannula is used to cut into the TM, which is visualized using gonioscopy. Once the microcatheter is in SC, it is advanced around the canal 180 degrees. The TM is torn as the microcatheter is taken out and the steps are repeated in the opposite direction to create a complete 360 degrees transluminal trabeculotomy.[27]
High-Frequency Deep Sclerostomy (HFDS)
HFDS utilizes the specialized Abee glaucoma tip (Oertli Instrument AG, Switzerland), a reusable high-frequency diathermic probe with a 15° posteriorly angled tip (1 mm length, 0.3 mm height, 0.6 mm width; 0.9 mm outer diameter) to create precise scleral-trabecular communication. The procedure begins with two 19-gauge MVR blade incisions (120° apart, upper temporal/nasal quadrants). After filling the anterior chamber with a high-density cohesive OVD, the abee tip is introduced via the Oertli OS3 phacoemulsification system and advanced under gonioscopic guidance to the trabecular meshwork (TM). Activation via foot pedal (confirmed by three audible beeps) drives the tip 1 mm into TM and SC, generating microbubbles as tissue dissipates. Six scleral pockets (0.3 mm deep × 0.6 mm wide) are created nasally, optimizing aqueous outflow while preserving structural integrity. The procedure concludes with OVD irrigation and incision hydration, ensuring minimal postoperative hypotony.[28] [29][30]
Surgical follow up
As with most glaucoma procedures, surgical follow-up after an AIT usually occurs at day 1, week 1, and then at the surgeon’s discretion depending on the patient’s progress. The immediate postoperative period normally entails checking the IOP and bleb appearance (if applicable). Presence of complications such as hyphema, infection, conjunctival/wound leak, shallow/flat AC, hypotony requiring intervention, and choroidal detachment should also be noted.
Complications
The ab interno approach has shown to have few complications and relatively less than traditional ab externo trabeculectomies,[3][7][8][13][27][31][32][33][34] with a complication rate of 4.3%, compared to 35.3% of traditional ab externo trabeculectomies.[33]
Intraoperative Complications
- Intraoperative bleeding is common, in particular in patients who are on antiplatelet agents, anti-coagulated, have clotting disturbances, or have an inflammatory condition. The mechanism for bleeding is blood reflux from episcleral vessels through the collecting channels into the unroofed Schlemm’s canal (SC) according to the pressure gradient.[35][36] The bleeding is usually more prominent when the intra-ocular pressure is lower than the venous pressure – while aspirating or evacuating the ophthalmic viscosurgical device (OVD), etc. Adding OVD may be beneficial. When a significant hyphema is present at the end of the procedure, IOP elevation might be needed. Use high-pressure infusion or surgical wound hydration with irrigation to increase the AC pressure. For good tamponade and prevention of continuous bleeding after the surgery, it is acceptable to leave some OVD in the anterior chamber (AC), instruct the patient to avoid heavy lifting and maintain a high head position.[17]
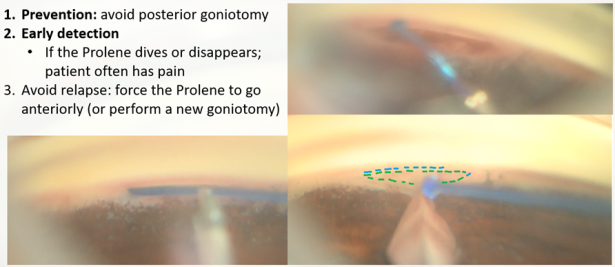
- Misplacement of the suture (GATT) The suture may be diverted posteriorly to the supra-choroidal space, which may cause pain to the patient and lead to supra-choroidal cleft, or anteriorly to the AC. In most cases, if the initiation of the nasal SC cannulation was successful, and advancement of the suture within the canal was observed, one may suspect resistance to suture advancement down the line (inner narrowing of the canal, external pressure on the canal, previously implanted device, or scarring). When the suture is misdirected, and a false passage is created, it may damage the adjacent tissues, creating cyclodialysis, iridiodialysis, etc. [37]The GATT procedure in this case will have a partial effect. If there is a need for further cannulating or unroofing of the SC, recannulation of the SC could be performed by entering the suture in the opposite direction.
- High resistance to suture advancement or a stop in suture advancement (GATT) A gentle redirection of the advancement trajectory can be tried, and advancing the trabeculotomy while centrally pulling the suture can be applied (“walk the dog” technique.) If these techniques are not helpful, a ripcord of the suture (unroofing) should be initiated. In extreme circumstances, one should consider advancing the suture in the opposite direction.
- Flattening of the anterior chamber can occur when OVD is evacuated from the AC during the procedure (with manipulation of surgical wounds, compression of the cornea with the gonioprism, or, rarely with a displacement of it to the vitreous cavity. If the AC remains flat despite refilling of cohesive OVD and removing any external pressure on the eye, a posterior pressure should be suspected and managed according to the cause (choroidal effusion, suprachoroidal hemorrhage, retrobulbar pressure, speculum pressing the globe, etc.)
- Descemet membrane injuries and detachment may be caused by surgical instruments, usually near the goniotomy site), in particular in narrow angles and when the eye is not adequately tilted or near the surgical entry site. This may lead to corneal edema or scarring. A rare incidence of Descemet detachment was reported with suture advancement[38], or rarely during viscodilation.
- Lens-related complications in phakic eyes, surgical instruments could accidentally touch, compress, or push the lens, and the anterior capsule may also be injured. In pseudophakic eyes, the AC is usually deeper, yet an IOL displacement may be caused, in the setting of unstable IOLs.[17]
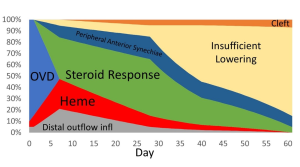 Differential diagnosis of IOP spike causes post-GATT. During the 1st day post-GATT, residual OVD is undoubtedly associated with the IOP spike, then hemorrhage and steroid response are the leading causes; later in the follow-up, we may consider insufficient IOP lowering from the surgery. (Image courtesy of Matthew Schlenker, MD)
Differential diagnosis of IOP spike causes post-GATT. During the 1st day post-GATT, residual OVD is undoubtedly associated with the IOP spike, then hemorrhage and steroid response are the leading causes; later in the follow-up, we may consider insufficient IOP lowering from the surgery. (Image courtesy of Matthew Schlenker, MD)
Postoperative Complications
- Bleeding e.g., microhyphema, hyphema, and vitreous hemorrhage are caused by blood reflux from the episcleral veins into the anterior chamber (AC). This could be explained by the pressure gradient caused by a relatively hypotonic AC (leaky surgical wounds, OVD evacuation or aspiration, and compression by the gonioprism or other surgical tools)[35]. Hyphema is a transient, self-resolving sequel rather than a complication since the correlation with long-term results is debatable – while in some studies hyphema was found to be a risk factor for developing an IOP spike, in others, it was not.[35] [39][40]Re-bleedings may occur and are not uncommon, usually within the first postoperative month. In rare cases, a AC washout or vitrectomy may be needed.
- IOP spike A transient IOP elevation, generally defined as IOP > 30 mmHg or >10% elevation from baseline pre-operative IOP. A spike may be seen during the first postoperative month but is more common between postoperative day 4 and the second postoperative weeks.[41] A spike is usually treated with an increasing dosage of hypotensive drugs in a stepwise manner, according to the severity of glaucoma and the spike. An anterior chamber tap may be considered a bridging therapy and may be repeated. Adding topical hypotensive agents, oral carbonic anhydrase, and systemic hyperosmotic drugs are generally the main path to control the spike. Rarely, a secondary glaucoma surgery is required (a cyclophotocoagulation, a tube-shunt, trabeculectomy, etc.
- Inflammation Iritis or anterior uveitis is usually well controlled with anti-inflammatory drugs – topical steroids, non-steroidal anti-inflammatory drugs, or both, with rapid tapering during the first month. Sometimes dosage adjustments or rate of tapering are needed, especially for patients with uveitic glaucoma, significant steroid response, or significant bleeding.
- Goniosynechiae or peripheral anterior synechiae (PAS) may form, in particular in cases of shallow AC (in primary angle closure, in phakic eye, or due to hypotony or choroidal effusion), in eyes with significant inflammation or predisposed eyes. Pilocarpine 2% is sometimes added to the postoperative regimen to avoid it. Laser iridoplasty may also be done in such cases. [42]
- Cystoid macular edema (CME) may be present after surgery, and as in pseudophakic CME (Irvine-Gass syndrome), is usually controlled with topical anti-inflammatory agents but may require intravitreal injections of corticosteroids or anti-VEGF agents.
- Wound leaks are uncommon. Usually, watchful monitoring is acceptable (with or without a bandage contact lens and or aqueous depressant drops addition), though sometimes suturing the surgical wounds is necessary.
- Infection or endophthalmitis is a severe and rare complication of any intraocular procedure.
- Descemet detachment An iatrogenic Descemet detachment may rarely occur (at the surgical wound or adjacent to the goniotomy), leading to scarring or corneal edema, temporary or permanent.[17]
- Shallow anterior chamber is infrequent; etiologies are similar to other glaucoma surgeries: cyclodialysis, choroidal effusion or hemorrhage (extremely rare), wound leak, and anterior synechiae.
- Hypotony is a rare complication and is usually secondary to an accidental creation of a cyclodialysis cleft during the procedure[37] or possibly ciliary body shutdown in predisposed eyes. Hypotony is IOP < 6 mmHg or low IOP with shallow AC, maculopathy, or choroidopathy. Post-procedure hypotony is usually self-resolving within days but may need intervention such as topical steroids and atropine or injection of viscoelastic (OVDs).
- Procedure failure. Lack of achieving surgical success (success definition varies among different studies, but in most cases defined as IOP < 21 mmHg and >20% reduction from baseline IOP or reduction in IOP-lowering drugs) at POM6, loss of light perception or need for a secondary IOP-lowering surgical procedure.[17][35]
Prognosis
Trabectome Outcomes
The overall success of Trabectome varies between studies and methods used. A meta-analysis showed the success rate--defined as IOP < 21, at least 20% decrease in IOP, and no further surgical intervention required for glaucoma up to two years following the initial surgery--of a Trabectome alone to be approximately 46%, and 85% when combined with phacoemulsification. The overall success rate was found to be approximately 66% at a two-year mark. In the Trabectome alone and Trabectome plus phacoemulsification groups, the reduction in IOP at two years was measured to be approximately 10.5 mm Hg (39%) and 6.24 mm Hg (27%), respectively. The number of medications needed to manage IOP was decreased by 0.99 and 0.76 medications, respectively.[31]
The ab interno approach has been directly compared to the more traditional ab externo trabeculectomy. In a retrospective cohort study, using the trabectome method for AIT, IOP was reduced by 43.5% in patients receiving AIT and 61.3% in ab externo trabeculectomy patients at two years. Overall, the success rate--defined as IOP <21 mm Hg and at least 20% decrease in IOP--of AIT was found to be 22.4% compared to 76.1% success rate yielded by ab externo trabeculectomies. Given the lower success rate, approximately 43.5% of patients required further surgical intervention to control IOP compared to 10.8% of patients receiving traditional trabeculectomies.[33]
Ab externo trabeculectomies are more commonly performed than ab interno trabeculectomies, however ab externo trabeculectomies can often fail to achieve IOP goals. Following the failure of an ab externo trabeculectomy surgery, an additional ab interno trabeculetomy can lower IOP and medications. In a prospective study, Trabectome was performed within one year of a failed ab externo trabeculectomy showed an IOP reduction by 28% and medication count decrease by approximately 0.8 medications. Trabectome combined with phacoemulsification in the same situation yields reductions of IOP by 19% and medications by roughly 0.9 medications. The overall success rates are 81% and 87%, respectively, for an Trabectome and Trabectome plus phacoemulsification one year following a failed trabeculectomy. [32]
KDB Outcomes
Goniotomy with KDB continues to gain acceptance and widespread use amongst clinicians because of its favorable risk profile compared to both filtering surgeries and other MIGS procedures in addition to the documented success rates. Studies published since it came onto the market in 2015 have demonstrated these benefits. Given the relative novelty of this blade however, most published studies are limited to 1 year of follow up.
A prospective multicenter study by Dorairaj et al. of 52 eyes that underwent phaco in combination with KDB showed an IOP reduction of 26.2% at 1-year follow-up (P<0.001). Pre-operative IOP average in this study was 16.8 ± 0.6 mm Hg at baseline and 12.4 ± 0.3 mm Hg at month 12.[43] The same cohort had 63.5% of patients with at least 1 medication reduction at month 12. In another multi-center study by Greenwood et al, 58.3% of patients had an IOP reduction of at least 20% and 61.7% were using at least 1 fewer IOP lowering medications after 6 months of follow up.[11] A retrospective review of 100 patients who underwent goniotomy either with or without phaco in adults showed a significant decrease in IOP in all eyes (from 16.5 ± 5.0 mm Hg to 14.4 ± 3.7 in the combined group and 24.3 ± 9.1 mm Hg to 16.7 ± 7.6 in the standalone group) without a significant difference between the two groups at 18 months (P =0.5) . [44]Another recent retrospective review of 197 eyes which underwent either KDB goniotomy alone or combined with phaco showed a significant decline in IOP and glaucoma medication usage at 1 year follow up. Success, defined as 20% decrease in IOP, was achieved in 71.8% of patients who underwent phaco-KDB and 68.8% of patients who underwent KDB goniotomy alone.[45]
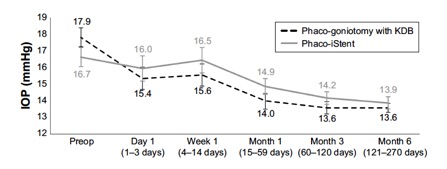
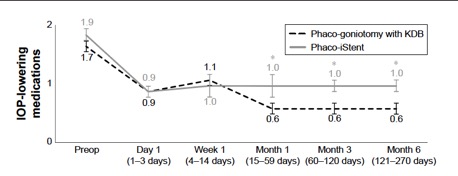
Several studies have compared results of phaco plus KDB goniotomy to phaco plus iStent procedures. The KDB Goniotomy Study Group demonstrated that their phaco-KDB cohort experienced a significantly greater decrease in IOP (23.7% vs 16.4%, p<0.001) and reduction in medication usage (1.1 vs 0.9 medications, p=0.001) when compared to the phaco-iStent group at 6 months follow up. [46]These results were further substantiated by a prospective randomized controlled trial by Falkenberry et al. comparing phaco-KDB and phaco-istent. At 12 months follow up, the primary outcome (20% or greater IOP reduction or medication reduction of 1 or more) was achieved by 74/79 patients (93.7%) in the phaco-KDB group and 65/78 patients (83.3%) in the phaco-iStent group (P =0.04). [47]Finally, a large retrospective analysis of 315 eyes by ElMallah et al. demonstrated that mean IOP reductions were significantly greater in their phaco-KDB group than in their phaco-iStent group at all time points including month 12 [- 5.0 (0.3) mmHg vs. - 2.3 (0.4) mmHg, respectively, p < 0.001].[48]
While the comparison between phaco plus KDB goniotomy and phaco plus iStent procedures has the most clinical data to date, there have been other studies comparing phaco plus KDB to other phaco-plus procedures. For example, Hirabayashi et al. compared phaco plus KDB to phaco plus 360° trabeculotomy (via either Trab360 or gonioscopy-assisted transluminal trabeculotomy [GATT]). They found that mean IOP reductions and medication reductions were similar between groups at 6 months. However, more eyes undergoing KDB than Trab360/GATT achieved target IOP ≤18 mm Hg (80.0% [56/70] vs 59.3% [16/27], P=0.040) and ≤15 mm Hg (61.4% [43/70] vs 25.9% [7/27], P=0.003).[18]
An important factor when considering any phaco plus procedure is its potential to impact refractive outcomes. Sieck et al. published a retrospective review of patients who received either phaco plus KDB or phaco alone and showed no significant difference in the rate of refractive surprise between the two groups. They found that refractive surprise greater than ±0.5 D occurred in 26.3% of eyes in the phaco-KDB group and 36.2% in the phacoemulsification group (p = 0.11). Refractive surprise greater than ±1.0 D occurred in 6.6% for the phaco-KDB group and 9.7% for the phacoemulsification group (p = 0.08). This data helps alleviate potential concerns regarding KDB’s impact on refractive outcomes when paired with cataract surgery.[49]
In a subpopulation of individuals suffering from severe and/or refractory glaucoma and undergoing Kahook Blade Goniotomy in standalone fashion or in combination with phacoemulsification, mean IOP decreased from 18.1 + 5.0 mm Hg at baseline to 14.8 + 3.7 mm Hg at 12 months (18.2% reduction, P<0.001).[50]
Of note, all of the studies mentioned have included patients with mild, moderate, and severe glaucoma, however few, if any studies have evaluated whether success rates differed amongst these groups. Similarly, the majority of patients in these studies had primary open-angle glaucoma, but cohorts also included patients with pigmentary, pseudoexfoliative, and angle closure glaucoma. Subgroup analyses were not routinely performed or were not of adequate power.[11] [12][43][45][46][51][52][53]
However, there have been recent studies investigating the use of KDB in unique clinical scenarios including CACG, uveitis, and severe refractory glaucoma. A retrospective analysis of 42 eyes with CACG undergoing phaco plus KDB showed IOP reduction of 12.3 mm Hg (p<0.0001) as well as a reduction of IOP-lowering medications by 2.2 (p<0.0001) at a 12 month follow up. Furthermore, 92.9% of eyes achieved IOP ≤18 mmHg, 100% achieved IOP reduction of ≥20%, 95.2% required ≥1 fewer medications for IOP control, and 85.7% (36/42) were medication-free.[54] In a small retrospective study of 16 uveitic eyes undergoing KDB either with or without phaco, Miller et al. showed 10 eyes (62.5%) maintained an IOP at or below their goal through their most recent follow-up visit (mean follow-up time of 9.6±5.6 months) and the mean number of glaucoma medications was significantly reduced by 1.5±1.4 medications (P=0.004).[55] Finally, a retrospective analysis of 53 eyes with severe or refractory glaucoma demonstrated an IOP reduction of >20% in 57.7% of eyes at 6 months. Furthermore, 63.5% and 92.3% of eyes achieved an IOP≤14 and ≤18 mm Hg, respectively, and the mean number of glaucoma medications was reduced by 1.2±1.3 (36.6%) compared with baseline (P<0.001).[56]
GATT Outcomes
GATT surgery has a promising success rate while sparing conjunctiva for future incisional surgery. Its success varies from 46% at 18 months to 89% at 2 years and 75% at 3 years[42], and a meta-analysis estimated a combined surgical success of 85%.[57] It is vital to note slight variations in the success definition across these studies; the authors recommend using the World Glaucoma Association Guidelines for glaucoma surgical trials for comparability.
Faria et al.[35] described a success rate of 87% at 12 months of GATT and 91% of combined cataract surgery (phaco-GATT) at 1 year in a cohort of POAG patients, with patients older than 60 years having a higher risk of failure (hazard ratio of 10.96; P=0.026). The mean IOP decreased from 24.9±8.5 to 12.1±2.1 mm Hg (P<0.001). Another study showed a smaller (63%)[58] success rate at 12 months, smaller in African patients (42%, P<0.05), but with a 44% IOP decrease. Differently, a retrospective study[59] showed inferior success in phaco-GATT compared with GATT at one year of follow-up, respectively 83.8% and 87.5%. Other studies showed similar[60] qualified success of 86.21% in phaco-GATT and 83.48% in GATT at 24 months.[61]
Although GATT is classically performed in mild to moderate glaucoma, studies showed its effectiveness also in advanced glaucoma (83.7% of success in moderate to advanced open-angle glaucoma at 19.4 months.[62])
In primary congenital glaucoma, performing GATT surgery avoided filtering surgery with a cumulative probability of qualified success of 95.5% and complete success of 66.7% at 1 year of follow-up[63]. Patients with juvenile open-angle glaucoma (JOAG) also had good results with GATT, even with severe JOAG, considering high complete and qualified success rates of 74.3% and 91.4% at 12 months[41] and 58.6% and 81.2% at 18 months.[64]
Patients with pseudoexfoliative glaucoma (PXG) are good candidates for GATT, with a higher success than POAG patients according to some studies[16], but similar according to a recent study[63], in which the cumulative success probability was higher during the first year in PXG (97.6%) than POAG (86.8%, P=0.01); but without no significant difference at 2-year nor 3-year visits (P=0.24). The success rate is high in PXG, 89.2% at 2 years follow-up.[65]
Uveitic glaucoma is one of the subgroups in which GATT is known for its relative success (81% of cumulative success at 12 months and a mean IOP decrease of 68%[66] [67])
GATT is also useful for secondary glaucoma after silicone oil[68] and steroid-induced glaucoma.[69][70]
In contrast, a combined phaco-GATT approach is preferred in primary closed-angle glaucoma (ACG). Still, a prospective study showed an acceptable success of 73% at 12 months[71] and 78% two years[72] after isolated GATT in PACG.
Trabeculectomy’s success has been described between 56-65% at five years (by the TVT study[73]) and 79% at five years (by the CIGTS study[74]), and trabeculectomy has many risks and complications that may be frequent (up to 50%) and potentially blinding.[75]
Studies comparing GATT with trabeculectomy show a trend toward higher success in trabeculectomy (59% Vs 46% complete success at 18 months in POAG, non-statistically significant, but statistically significant difference in % of IOP lowering[76]), despite the higher risk of complications with the latter. Comparing GATT with XEN[73], a retrospective study found superior results with GATT at 24 months (complete surgical success of 34.2% in XEN and 50.5% in GATT groups, p = 0.039; and qualified success of 97.4% in XEN and 89.7% in GATT, p=0.025).
Comparing GATT with conventional trabeculotomy (CT), a retrospective study[77] showed a far superior success in phaco-GATT versus phaco-CT at 2 years (40.4% Vs 96.6%, p < 0.001) but a more negligible difference in isolated GATT versus CT (40.8% and 54.2%, p=0.55), which could indicate a synergic effect of phaco with GATT (as described with other MIGS).
Unsurprisingly, studies comparing phaco-GATT with phaco-iStent showed higher success in the first group ( success of 86.4% Vs. 35.1% at 1 year.)[78]
GATT was effective even in eyes with previously failed trabeculectomies, with a success rate of 60-70% at 24 months (and a cumulative proportion of reoperation of 0.29)[16], and of 61.5% in a case series[79] with 17.8±4.1 months (patients with PXG had more mean IOP lowering after GATT than patients with POAG, 45.6% Vs. 34.8%), among other studies with similar results.[80]
Omni Outcomes
In a retrospective study, the efficacy and safety profile of TRAB™360 (Omni predicate device) procedures performed on patients with refractory primary open-angle glaucoma and a pre-operative IOP of at least 18 mm Hg was explored. Over the first 12 months following a TRAB™360, approximately 59% of patients showed at least a 20% reduction in intraocular pressure and IOP less than 18 mm Hg, with the average number of medications dropping from 1.7 +/- 1.3 to 1.1 +/- 1.0 medications. Results were also promising in patients with even higher baseline IOP (at least 25 mm Hg) with 67% experiencing success. There was a 25% failure rate (patient needed further intervention within the first 12 months) and mild adverse effects with patients mostly experience transient hyphema.[32]
HFDS outcomes
open-angle glaucoma cases demonstrated significant and sustained reductions in intraocular pressure (IOP) following surgery. Mean IOP dropped from 24.5 ± 4.3 mm Hg preoperatively to 15.0 ± 1.7 mm Hg at 48 months (p < 0.001), with 84.9% achieving IOP <21 mm Hg and a 100% qualified success rate (IOP <22 mm Hg with medication). Visual acuity improved from 0.46 ± 0.27 to 0.68 ± 0.27, while visual fields and optic disc excavation showed no significant changes. Pressure-reducing medications decreased markedly from 2.6 ± 1.0 preoperatively to 0.48 ± 0.97 at 48 months. Complications were rare, with temporary IOP elevation in 8.7% of eyes and transient fibrin formation in 2% .[29]
Conclusion
Ab interno trabeculectomy and trabeculotomy procedures are a subtype of surgeries that fall under the umbrella of micro-invasive glaucoma surgery (MIGS). The ab interno approach has shown to be effective in a majority of patients when data is analyzed from several different studies looking at individual types of AIT surgeries.[3][7][8][13][27][31][32][33][34] When compared to traditional ab externo trabeculectomy, results have generally shown that AIT procedures yield an inferior efficacy.[33] However, these surgeries are still significantly effective and do not carry the same risk profile as traditional glaucoma surgeries, with complications outside of transient hyphema being extremely rare. [3][7][8][13][27][31][32][33] [34] Most studies are limited to 12-24 months following surgery, and it would be interesting to see patient outcomes over a longer duration.
References
- ↑ Poitras V, Wells C, Hutnik C, et al. Introduction. In: Optimal Use of Minimally Invasive Glaucoma Surgery: A Health Technology Assessment [Internet]. Canadian Agency for Drugs and Technologies in Health; 2019.
- ↑ Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012 Mar;23(2):96-104. doi: 10.1097/ICU.0b013e32834ff1e7. Review. PubMed PMID: 22249233.
- ↑ 3.0 3.1 3.2 3.3 Poitras V, Wells C, Hutnik C, et al. Minimally Invasive Glaucoma Surgery Devices and Procedures of Interest. In: Optimal Use of Minimally Invasive Glaucoma Surgery: A Health Technology Assessment [Internet]. Canadian Agency for Drugs and Technologies in Health; 2019.
- ↑ Baerveldt, G. and Chuck, R. “Minimally Invasive Glaucoma Surgical Instrument and Method” US Patent (2005): Available at https://www.google.com/patents/US6979328
- ↑ 5.0 5.1 Minckler, D., Baerveldt, G., Ramirez, M. A., Mosaed, S., Wilson, R., Shaarawy, T., Zack, B., Dustin, L., and Francis, B. “Clinical Results with the Trabectome, a Novel Surgical Device for Treatment of Open-Angle Glaucoma” Transactions of the American Ophthalmological Society 104, (2006): 40–50.
- ↑ 6.0 6.1 6.2 6.3 Kaplowitz, K., Schuman, J. S., and Loewen, N. A. “Techniques and Outcomes of Minimally Invasive Trabecular Ablation and Bypass Surgery” The British journal of ophthalmology 98, no. 5 (2014): 579–585.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013 Mar;155(3):524-529.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 SooHoo JR, Seibold LK, Kahook MY. Ab interno trabeculectomy in the adult patient. Middle East Afr J Ophthalmol. 2015;22(1):25–29.
- ↑ Arimura S, Iwasaki K, Orii Y, Komori R, Takamura Y, Inatani M. Randomised clinical trial for morphological changes of trabecular meshwork between Kahook dual-blade goniotomy and ab interno trabeculotomy with a microhook. Sci Rep. 2023 Nov 27;13(1):20783. doi: 10.1038/s41598-023-48121-5. PMID: 38012358; PMCID: PMC10682418.
- ↑ Epstein, RS, Soohoo, JS, Pantcheva, MP, Seibold, LK, Kahook, MY. Optimal time for visualization with minimally invasive Glaucoma Surgeries: Before or After Phacoemulsification? Data presented as a poster at the American Glaucoma Society 27th Annual Meeting; March 2017, Coronado, California.
- ↑ 11.0 11.1 11.2 Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: Short-term results. J Cataract Refract Surg. 2017;43(9):1197-1201.
- ↑ 12.0 12.1 Radcliffe, N, et al. A Novel Dual Blade Device for Goniotomy: Six-month Follow-up. Data presented as a poster at the American Glaucoma Society 27th Annual Meeting; March 2017; Coronado, California.
- ↑ 13.0 13.1 13.2 13.3 13.4 Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014 Apr;121(4):855-61
- ↑ Smith, R., Nylon filament trabeculotomy. Comparison with the results of conventional drainage operations in glaucoma simplex. Trans Ophthalmol Soc N Z, 1969. 21: p. 15-26.
- ↑ Chin, S., et al., Reduction of intraocular pressure using a modified 360-degree suture trabeculotomy technique in primary and secondary open-angle glaucoma: a pilot study. J Glaucoma, 2012. 21(6): p. 401-7
- ↑ 16.0 16.1 16.2 Grover, D.S., et al., Outcomes of Gonioscopy-assisted Transluminal Trabeculotomy (GATT) in Eyes With Prior Incisional Glaucoma Surgery. J Glaucoma, 2017. 26(1): p. 41-45.
- ↑ 17.0 17.1 17.2 17.3 17.4 Grover, D.S., et al., Gonioscopy-assisted Transluminal Trabeculotomy: An Ab Interno Circumferential Trabeculotomy: 24 Months Follow-up. J Glaucoma, 2018. 27(5): p. 393-401.
- ↑ 18.0 18.1 18.2 Hirabayashi MT, Lee D, King JT, Thomsen S, An JA. Comparison Of Surgical Outcomes Of 360° Circumferential Trabeculotomy Versus Sectoral Excisional Goniotomy With The Kahook Dual Blade At 6 Months. Clin Ophthalmol. 2019 Oct 15;13:2017-2024.
- ↑ Sato, T. and T. Kawaji, 12-month randomised trial of 360 degrees and 180 degrees Schlemm's canal incisions in suture trabeculotomy ab interno for open-angle glaucoma. Br J Ophthalmol, 2021. 105(8): p. 1094-1098.
- ↑ Okada, N., et al., Comparison of Efficacy between 120 degrees and 180 degrees Schlemm's Canal Incision Microhook Ab Interno Trabeculotomy. J Clin Med, 2021. 10(14).
- ↑ Alnahrawy, O., et al., Exit strategies in canaloplasty: intraoperative conversion into 180-degree trabeculotomy or 360-degree trabeculotomy in cases of unsuccessful catheterisation of Schlemm's canal: influence of degree of canal cleavage. Graefes Arch Clin Exp Ophthalmol, 2015. 253(5): p. 779-84
- ↑ 22.0 22.1 Alnahrawy, O., et al., Exit strategies in canaloplasty: intraoperative conversion into 180-degree trabeculotomy or 360-degree trabeculotomy in cases of unsuccessful catheterisation of Schlemm's canal: influence of degree of canal cleavage. Graefes Arch Clin Exp Ophthalmol, 2015. 253(5): p. 779-84.
- ↑ Carreon, T.A., et al., Segmental outflow of aqueous humor in mouse and human. Exp Eye Res, 2017. 158: p. 59-66.
- ↑ 24.0 24.1 Zeppa, L., et al., In Vivo Near-Infrared Fluorescence Imaging of Aqueous Humor Outflow Structures. J Ophthalmol, 2016. 2016: p. 8706564.
- ↑ Waldner, D.M., et al., Segmental Suture Gonioscopy-Assisted Transluminal Trabeculotomy: Comparison of Superior Versus Inferior Hemisphere Outcomes. J Glaucoma, 2023. 32(5): p. 396-406.
- ↑ Zhang, X., et al., In Vivo Imaging of Schlemm's Canal and Limbal Vascular Network in Mouse Using Visible-Light OCT. Invest Ophthalmol Vis Sci, 2020. 61(2): p. 23.
- ↑ 27.0 27.1 27.2 27.3 Sarkisian SR, Mathews B, Ding K, Patel A, Nicek Z. 360° ab-interno trabeculotomy in refractory primary open-angle glaucoma. Clin Ophthalmol. 2019;13:161–168.
- ↑ Abushanab MM, El-Shiaty A, El-Beltagi T, Hassan Salah S. The Efficacy and Safety of High‐Frequency Deep Sclerotomy in Treatment of Chronic Open‐Angle Glaucoma Patients. BioMed Research International. 2019;2019(1):1850141.
- ↑ 29.0 29.1 Pajic B, Cvejic Z, Mansouri K, Resan M, Allemann R. High-frequency deep sclerotomy, a minimal invasive Ab interno glaucoma procedure combined with cataract surgery: Physical properties and clinical outcome. Applied Sciences. 2019 Dec 27;10(1):218.
- ↑ Kontic M, Todorovic D, Zecevic R, Vulovic TS. High-Frequency deep sclerotomy as adjunctive therapy in open-angle glaucoma patients. Ophthalmic Research. 2023 Dec 20;66(1):339-44.
- ↑ 31.0 31.1 31.2 31.3 Kaplowitz K, Bussel II, Honkanen R, et alReview and meta-analysis of ab-interno trabeculectomy outcomesBritish Journal of Ophthalmology 2016;100:594-600.
- ↑ 32.0 32.1 32.2 32.3 32.4 Bussel II, Kaplowitz K, Schuman JS, et al. Outcomes of ab interno trabeculectomy with the trabectome after failed trabeculectomy. British Journal of Ophthalmology 2015;99:258-262.
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 Jea SY, Francis BA, Vakili G, Filippopoulos T, Rhee DJ. Ab interno trabeculectomy versus trabeculectomy for open-angle glaucoma. Ophthalmology. 2012 Jan;119(1):36-42.
- ↑ 34.0 34.1 34.2 Francis BA, See RF, Rao NA, Minckler DS, Baerveldt G. Ab Interno Trabeculectomy: Development of a Novel Device (Trabectome™) and Surgery for Open-Angle Glaucoma. Journal of Glaucoma. 2006; 15 (1): 68-73.
- ↑ 35.0 35.1 35.2 35.3 35.4 Faria, B.M., et al., Gonioscopy-Assisted Transluminal Trabeculotomy for Glaucoma: 1-Year Outcomes and Success Predictors. J Glaucoma, 2022. 31(6): p. 443-448.
- ↑ Velamala, I.P. and M. Bharathi, Gonioscopy Assisted Transluminal Trabeculotomy:A Boon to Developing Nations-A Systematic Review. Semin Ophthalmol, 2023. 38(2): p. 178-182.
- ↑ 37.0 37.1 Areaux, R.G., Jr., et al., Trabeculotomy Ab Interno With the Trab360 Device for Childhood Glaucomas. Am J Ophthalmol, 2020. 209: p. 178-186.
- ↑ Donmez Gun, R., et al., Partial Schlemm Canal, Trabecular Meshwork, and Descemet Membrane Separation During Gonioscopy-assisted Transluminal Trabeculotomy: A Case Report. J Glaucoma, 2020. 29(1): p. e1-e2.
- ↑ Shi, Y., et al., A Prospective Study of Intraocular Pressure Spike and Failure After Gonioscopy-Assisted Transluminal Trabeculotomy in Juvenile Open-Angle Glaucoma: A Prospective Study of GATT in JOAG. Am J Ophthalmol, 2022. 236: p. 79-88.
- ↑ Chen, J., et al., Risk Factors for Complications and Failure after Gonioscopy-Assisted Transluminal Trabeculotomy in a Young Cohort. Ophthalmol Glaucoma, 2020. 3(3): p. 190-195.
- ↑ 41.0 41.1 Faria, B.M., et al., Short-term endothelial cell density changes after gonioscopy-assisted transluminal trabeculotomy. Arq Bras Oftalmol, 2021. 85(4): p. 344-350.
- ↑ 42.0 42.1 Sato, T., A. Hirata, and T. Mizoguchi, Prospective, noncomparative, nonrandomized case study of short-term outcomes of 360 degrees suture trabeculotomy ab interno in patients with open-angle glaucoma. Clin Ophthalmol, 2015. 9: p. 63-8.
- ↑ 43.0 43.1 Dorairaj SK, et al. 12-Month Outcomes of Goniotomy Performed Using the Kahook Dual Blade Combined with Cataract Surgery in Eyes with Medically Treated Glaucoma. Adv Ther. 2018 Sep;35(9):1460-1469.
- ↑ Wakil SM, et al. Efficacy and safety of a single-use dual blade goniotomy: 18-month results. J Cataract Refract Surg. 2020 Oct;46(10):1408-1415.
- ↑ 45.0 45.1 Sieck EG, et al. Outcomes of Kahook Dual Blade Goniotomy with and without Phacoemulsification Cataract Extraction. Ophthalmol Glaucoma. 2018 Jul-Aug;1(1):75-81.
- ↑ 46.0 46.1 Dorairaj SK, et al. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clin Ophthalmol. 2018;12:791-797.
- ↑ Falkenberry S, et al. Excisional goniotomy vs trabecular microbypass stent implantation: a prospective randomized clinical trial in eyes with mild to moderate open-angle glaucoma. J Cataract Refract Surg. 2020 Aug;46(8):1165-1171.
- ↑ ElMallah MK, Seibold LK, Kahook MY, Williamson BK, Singh IP, Dorairaj SK; KDB Goniotomy Study Group. 12-Month Retrospective Comparison of Kahook Dual Blade Excisional Goniotomy with Istent Trabecular Bypass Device Implantation in Glaucomatous Eyes at the Time of Cataract Surgery. Adv Ther. 2019 Sep;36(9):2515-2527.
- ↑ Sieck EG, et al. Refractive outcomes among glaucoma patients undergoing phacoemulsification cataract extraction with and without Kahook Dual Blade goniotomy. Eye Vis (Lond). 2019 Sep 19;6:28.
- ↑ Bravetti GE, et al. Surgical outcomes of excisional goniotomy using the kahook dual blade in severe and refractory glaucoma: 12-month results. Eye (Lond) 2022.
- ↑ Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34(7):1096-103.
- ↑ Abdullah S, Jasek MC, Radcliffe NM, et al. A novel dual blade device for goniotomy: initial clinical experience. Invest Ophthalmol Vis Sci. 2016;57(12):6522.
- ↑ Mansouri K, et al. Comparison of the Intraocular Pressure Lowering Efficacy Between a Novel Goniotomy Blade and a Trabecular Meshwork Device. Data presented as a poster at the XXXV Congress of the European Society of Cataract and Refractive Surgeons (ESCRS); October 7-11, 2017, Lisbon, Portugal.
- ↑ Dorairaj S, Tam MD, Balasubramani GK. Twelve-month outcomes of excisional goniotomy using the Kahook Dual Blade® in eyes with angle-closure glaucoma. Clin Ophthalmol. 2019 Sep 10;13:1779-1785.
- ↑ Miller VJ, Young CEC, SooHoo JR, Seibold LK, Kahook MY, Pecen PE, Palestine AG, Pantcheva MB. Efficacy of Goniotomy With Kahook Dual Blade in Patients With Uveitis-associated Ocular Hypertension. J Glaucoma. 2019 Aug;28(8):744-748.
- ↑ Salinas L, Chaudhary A, Berdahl JP, Lazcano-Gomez GS, Williamson BK, Dorairaj SK, Seibold LK, Smith S, Aref AA, Darlington JK, Jimenez-Roman J, Mahootchi A, Boucekine M, Mansouri K. Goniotomy Using the Kahook Dual Blade in Severe and Refractory Glaucoma: 6-Month Outcomes. J Glaucoma. 2018 Oct;27(10):849-855.
- ↑ Guo, C.Y., X.H. Qi, and J.M. Qi, Systematic review and Meta-analysis of treating open angle glaucoma with gonioscopy-assisted transluminal trabeculotomy. Int J Ophthalmol, 2020. 13(2): p. 317-324.
- ↑ Rahmatnejad, K., et al., Surgical Outcomes of Gonioscopy-assisted Transluminal Trabeculotomy (GATT) in Patients With Open-angle Glaucoma. J Glaucoma, 2017. 26(12): p. 1137-1143.
- ↑ Bozkurt, E., et al., The efficacy of gonioscopy-assisted transluminal trabeculectomy combined with phacoemulsification. Int Ophthalmol, 2021. 41(1): p. 35-43.
- ↑ Bektas, C., et al., Prognostic factors affecting the surgical success of gonioscopy-assisted transluminal trabeculotomy. Indian J Ophthalmol, 2021. 69(6): p. 1425-1429.
- ↑ Wan, Y., et al., Gonioscopy-assisted Transluminal Trabeculotomy (GATT) combined phacoemulsification surgery: Outcomes at a 2-year follow-up. Eye (Lond), 2023. 37(6): p. 1258-1263.
- ↑ Aktas, Z., et al., Surgical Outcomes of Prolene Gonioscopy-assisted Transluminal Trabeculotomy in Patients With Moderate to Advanced Open-Angle Glaucoma. J Glaucoma, 2019. 28(10): p. 884-888.
- ↑ 63.0 63.1 Aktas, Z., et al., Outcomes of Prolene Gonioscopy Assisted Transluminal Trabeculotomy in Primary Open Angle Glaucoma and Pseudoexfoliation Glaucoma: A Comparative Study. J Glaucoma, 2022. 31(9): p. 751-756.
- ↑ Wang, Y., et al., Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. Eye (Lond), 2021. 35(10): p. 2848-2854.
- ↑ Sharkawi, E., et al., Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. Br J Ophthalmol, 2021. 105(7): p. 977-982.
- ↑ Belkin, A., et al., Gonioscopy-assisted transluminal trabeculotomy is an effective surgical treatment for uveitic glaucoma. Br J Ophthalmol, 2023. 107(5): p. 690-697.
- ↑ Parikh, D.A., et al., Gonioscopy-Assisted Transluminal Trabeculotomy for the Treatment of Glaucoma in Uveitic Eyes. Ocul Immunol Inflamm, 2022: p. 1-7.
- ↑ Aktas, Z., et al., Outcomes of Gonioscopy-assisted Transluminal Trabeculotomy in Vitrectomized Patients With Secondary Glaucoma After Silicone Oil Removal. J Glaucoma, 2021. 30(3): p. e114-e118.
- ↑ Boese, E.A. and M. Shah, Gonioscopy-assisted Transluminal Trabeculotomy (GATT) is An Effective Procedure for Steroid-induced Glaucoma. J Glaucoma, 2019. 28(9): p. 803-807.
- ↑ Iwao, K., et al., Success rates of trabeculotomy for steroid-induced glaucoma: a comparative, multicenter, retrospective cohort study. Am J Ophthalmol, 2011. 151(6): p. 1047-1056 e1.
- ↑ Fontana, L., et al., Gonioscopy-assisted transluminal trabeculotomy for chronic angle-closure glaucoma: preliminary results. Graefes Arch Clin Exp Ophthalmol, 2022. 260(2): p. 545-551.
- ↑ Sharkawi, E., et al., Gonioscopy-assisted transluminal trabeculotomy in primary angle-closure glaucoma. Graefes Arch Clin Exp Ophthalmol, 2021. 259(10): p. 3019-3026.
- ↑ 73.0 73.1 Olgun, A., Z. Aktas, and A.Y. Ucgul, XEN gel implant versus gonioscopy-assisted transluminal trabeculotomy for the treatment of open-angle glaucoma. Int Ophthalmol, 2020. 40(5): p. 1085-1093.
- ↑ Jampel, H.D., et al., Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol, 2005. 140(1): p. 16-22.
- ↑ Investigators, A., The Advanced Glaucoma Intervention Study (AGIS): 11. Risk factors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol, 2002. 134(4): p. 481-98.
- ↑ Fontana, L., et al., Comparison of Gonioscopy-assisted Transluminal Trabeculotomy Versus Trabeculectomy With Mitomycin C in Patients With Open-angle Glaucoma. J Glaucoma, 2021. 30(1): p. 101-108.
- ↑ Takata M, Ishikawa H, Ikeda T, Gomi F. Conventional Trabeculotomy versus Gonioscopy-Assisted Transluminal Trabeculotomy: A Retrospective Cohort Study. J Clin Med. 2021 Dec 23;11(1):46. doi: 10.3390/jcm11010046. PMID: 35011786; PMCID: PMC8745353.
- ↑ Hamze, H., et al., Comparison of 1-year surgical outcomes of combined cataract surgery and gonioscopy-assisted transluminal trabeculotomy (GATT) versus cataract surgery and iStent Inject. Graefes Arch Clin Exp Ophthalmol, 2021.
- ↑ Cubuk, M.O., A.Y. Ucgul, and E. Unsal, Gonioscopy-assisted transluminal trabeculotomy as an option after failed trabeculectomy. Int Ophthalmol, 2020. 40(8): p. 1923-1930.
- ↑ Wang, Y., et al., Gonioscopy-assisted transluminal trabeculotomy for open-angle glaucoma with failed incisional glaucoma surgery: two-year results. BMC Ophthalmol, 2023. 23(1): p. 89.