Rhino-Orbital-Cerebral Mucormycosis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Rhino-orbital-cerebral-mucormycosis (ROCM), previously referred to as orbital zygomycosis, refers to the presentation of pathologic symptoms in the orbit as a result of fungal infections caused by fungi in the order Mucorales, most commonly by the species Rhizopus oryzae. ROCM usually occurs in an immunocompromised host and presents with initial symptoms such as vision loss, ptosis, diplopia, and external ophthalmoplegia. Left untreated, ROCM can progress to acute vision loss, metastasis (brain, sinuses) and death. [1][2]
Etiology
The primary species responsible for the development of ROCM is the fungus Rhizopus oryzae. This fungus is a fast growing, aseptate filamentous fungus that is responsible for nearly 90% of all rhinocerebral cases and has an overall mortality rate of over 50%. [3][2] Rhizopus oryzae is commonly found in tropical and subtropical regions, such as Australia, India, and the coastal parts of Texas.[4] Like most diseases of fungal origin, mucormycosis is usually introduced to the body through inhalation of spores. The two characteristics most often responsible for the proliferation and development include its propensity for induction of angiogenesis and rapid growth rate.
Risk Factors
Risk Factors: [5]
- Diabetes Mellitus II, particularly with diabetic ketoacidosis
- History of Kidney Transplant [6]
- Immunosuppressant therapy
- Intravenous Drug Users
- Human immunodeficiency virus (HIV) infection
- Neutropenia
- Hemochromatosis
- Deferoxamine therapy
Pathophysiology
Ketoacidosis is a very common predisposing factor for invasion and spread by mucor. Under acidotic conditions, iron fails to bind to its sequestering proteins like transferrin and becomes available to invading pathogens like mucor, which depend on iron for survival. This makes ketoacidosis a very susceptible predisposing condition for development of ROCM. [7] This is also the mechanism for iron overload conditions to cause ROCM (e.g., hemochromatosis, multiple blood transfusions, desferrioxamine). Mucormycosis commonly begins after the paranasal inhalation of spores and the formation of coenocytic hyphae that can spread. Once inhaled, they initially proliferate in the sinuses and can find their way to the orbit either through direct invasion or via the nasolacrimal duct. It can gain access to the brain either via spread from the orbital apex, cavernous sinus, through the cribriform plate, or through the blood vessels.
The primary mechanism of spread is through angioinvasion of blood vessels: vasotropic. It causes thrombosis, ischemia and disseminates necrosis with poor bleeding. Therefore, frequently in orbital invasion the eye lacks of the typical “hyperemia” (redness) of infection. The angioinvasion also gives the fungus access to additional heme as a source of iron. [7]
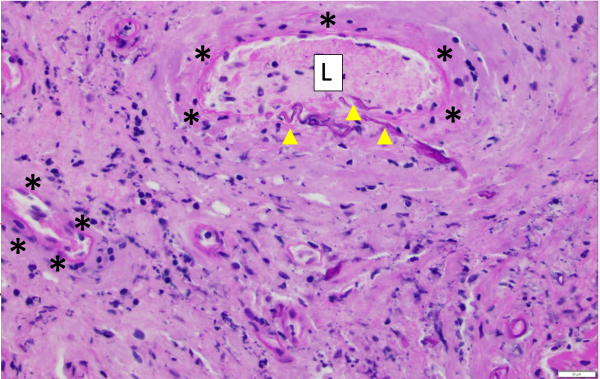
One critical step that allows for angioinvasion to occur is the penetration of and damage to the endothelial cells and extracellular matrix proteins lining the blood vessels. One receptor that is known to be involved in the penetration process is a protein known as glucose-regulated protein (GRP78). [7]
Once spread to the orbit, the disease is classified as ROCM. Initial ophthalmic problems presenting in ROCM are due to the tissue inflammation resulting from necrosis of the adjacent tissue to the orbit and reduced blood flow. Potentially serious ophthalmic problems associated with ROCM include occlusion of the central artery of the retina and infarction of the orbit including the optic nerve and can ultimately cause complete vision loss. From the orbit, ROCM can spread to the brain through the cribriform plate and orbital apex, causing potential complications such as internal carotid artery occlusion, cranial nerve palsy, chiasmal infarction, intracranial aneurysm, fungal meningitis, and even death. [1][8][9]
Primary Prevention
A chronic immunocompromised state is the most common predisposing condition for ROCM (e.g. HIV, diabetes mellitus II, organ transplant recipients taking immunosuppressants, deferoxamine treatment).
Diagnosis
Signs & Symptoms
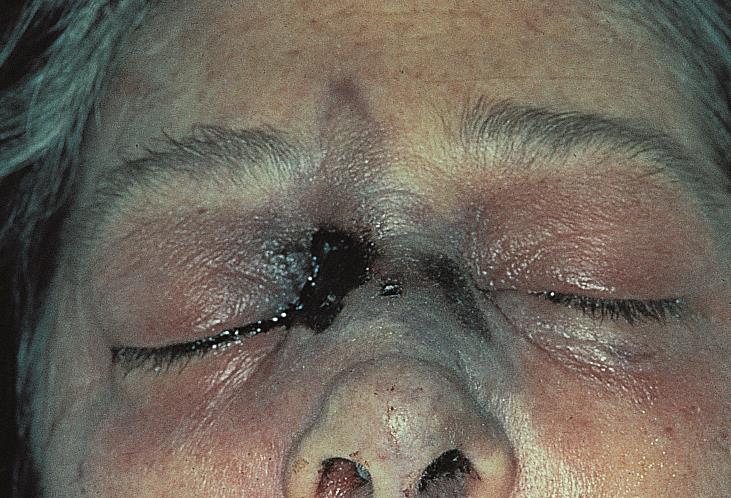
The early clinical signs of Mucormycosis include sinusitis, nasal discharge, and epistaxis. The hallmark of spread beyond the sinuses involve a characteristic black eschar over the skin over the orbit, palate, and nasal mucosa.[4] The eschar may propagate to the surrounding skin.
The maxillary and ethmoid sinuses are more frequently involved as evidenced on CT/MRI studies [8]. Initial external ophthalmic signs of ROCM may include the following [1][8] with ophthalmoplegia and proptosis being the most common eye signs reported [9][10].
- Periorbital Edema
- Cellulitis
- External Ophthalmoplegia or Frozen Globe (critical sign)[11][12]
- Proptosis
- Ptosis
- Orbital apex syndrome (Initial presentation of OAS, which often presents with frozen globe, may indicate advanced spread of infection due to extensive cranial nerve involvement with high mortality)[11][12][13]
- Eyelid gangrene
- Cavernous sinus thrombosis
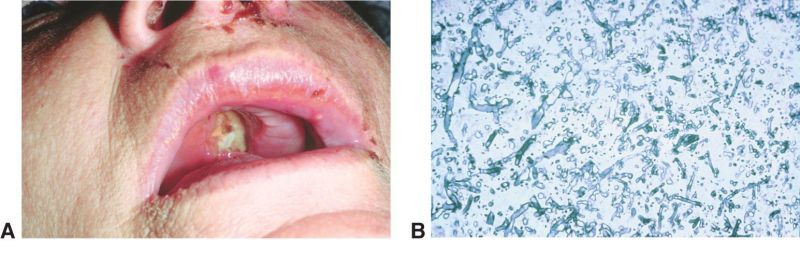
- Nasal and oral mucosa gangrene are seen as a black eschar, therefore it is important to also assess the oral and nasal mucosa of patients
Systemic
- Malaise
- Fever
There can be occlusion of the central artery of the retina, or the branch retinal arteries. Funduscopic exam typically reveals opacification of the superficial retinal layers and the characteristic cherry-red spot on the macula.[14] Rarely, vision loss can also occur from infarction or direct fungal invasion of the optic nerve or chiasm. [15][1]
Another manifestation of mucormycosis in the nasal-orbit area is a black eschar that may present in the nose mucosa or upper palate. Therefore, careful examination of these areas are advised when ROCM is suspected.
Diagnostic procedures
It is important to notice that because the fungus stays predominately intravascular, a CT scan without contrast may not reveal any lesion if no mass is formed, but may however show bone erosion if profound.
Imaging studies may include CT/MRI of the orbit, brain, and sinuses which may demonstrate involvement of the maxillary and ethmoid sinus, orbit, cavernous sinus, and less frequently, the frontal and sphenoid sinus. In order to obtain a true diagnosis of mucormycosis, a fine needle aspiration biopsy of infected tissues must be obtained for histopathology and culture. Characteristic of this disease is aseptate hyphal elements that branch at right angles. [16]
The most common areas to be involved are nasal cavity, maxillary sinus, ethmoid sinus, and orbit. [17]
On MRI, it is common to see isointense lesions (when compared to brain) in T1-weighted images. Most of the patients show hypointense T2-weighted images.[18] When the cavernous sinus is involved, MRI will show “lack of enhancement”.
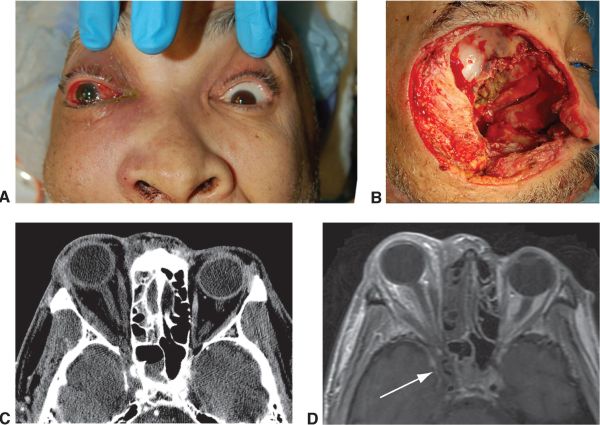
The case below is an example where mucormycosis extended into the orbit, optic nerve, and intracranially near the chiasm which was partially seen on CT and well-highlighted by orbital and brain MRI. The surgical approach was planned based on the images:
Some of the signs noted on a study of 43 patients with mucormycosis in the midface and skull base included: [19]
- No sinus wall destruction
- Inflammatory changes
- Cavernous sinus and the carotid artery involvement
- Isolated hard palate only (one patient)
Finally, the gold standard diagnostic procedure is biopsy and culture. Histopathological confirmation included H+E (hematoxylin-eosin), PAS (periodic acid-Schiff) and GMS (Grocott-Gomori's methenamine silver stain), among others.
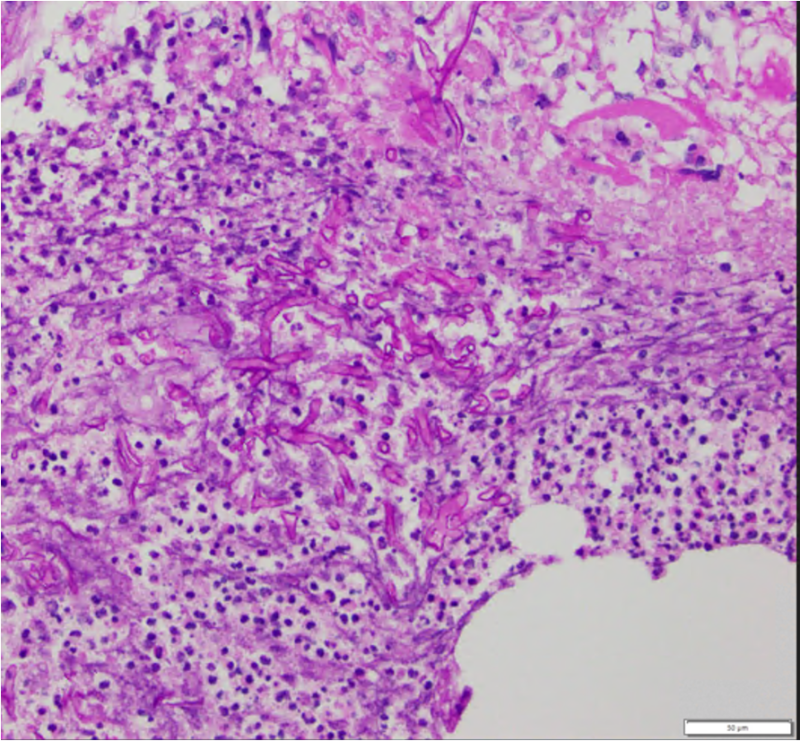
Differential diagnosis
Main differential is Aspergillus.
Ruling out Aspergillus and other fungi, is essential for diagnosis of ROCM. Circulating antigen detection test and 1,3 beta-D-glucan detection test cannot confirm the distinction between the Aspergillus and Rhizopus Oryzae. In conditions when tissue biopsy cannot be obtained due to the patient's condition, samples such as sputum or even bronchoalveolar lavage may allow for delineation between the two. [20] Biopsy of Aspergillus will demonstrate a 45-degree branching dichotomous (two, symmetric, almost-equal branches) septate fungus.
Management
General treatment
The standard treatment for the early stages of ROCM is antifungal therapy and debridement (e.g., liposomal amphotericin B), [21] and if localized, may be done endoscopically. Combination therapy of amphotericin with capsofungin has shown to be associated with better outcomes and can be considered. [2]
As a general rule, the goal of surgery is the removal of all visible necrotic tissues. Since necrotic tissue does not usually bleed, the surgical endpoint is to debride until normal or profuse bleeding is obtained. [22] In the event of spread to the craniofacial tissues and the orbit, surgical treatment becomes essential. Because mucormycosis spreads through blood vessels and tissues, surgical debridement of infected sites is considered to avoid further complications.
The surgical approach may vary. The presence of extensive orbital involvement may require orbital exenteration with bone removal. The extent of extra-orbital excision may be guided by imaging (i.e. MRI). Once the infection evolves into ROCM and the patient demonstrates symptoms consistent with deep orbital involvement, orbital exenteration may be considered to avoid the more lethal sequelae of ROCM when intracranial vessels, such as the Circle of Willis is affected. [23] Liposomal amphotericin B infusion can be continued throughout the course of surgical treatment. [24]
Note: It is highly recommended for the surgeon to identify and label the margins excised during surgery, so that the pathologist can rule out/in existing invasion and confirm remaining "healthy margins".
Prognosis
The long-term outlook for patients with ROCM depends on the severity of disease dissemination and the sites involved. Medical and surgical treatment of the ROCM, as well as the underlying immunosuppressive conditions are associated with better survival rates. In conducted studies in recent years, overall survival is 59.5% with treatment and only 21% without. [25] Factors associated with poor prognosis include: [9]
- Delay in diagnosis and initiation of treatment
- Treatment with amphotericin B alone
- Eyelid/facial gangrene
- Intracranial/intracranial invasion
- Hemiplegia
References
- ↑ 1.0 1.1 1.2 1.3 Mukherjee, B., Raichura, N. D., & Alam, M. S. (2016). Fungal infections of the orbit. Indian journal of Ophthalmology, 64(5), 337-45
- ↑ 2.0 2.1 2.2 Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005 Sep 1;41(5):634-53
- ↑ Ma L-J, Ibrahim AS, Skory C, Grabherr MG, Burger G, Butler M, et al. (2009) Genomic Analysis of the Basal Lineage Fungus Rhizopus oryzae Reveals a Whole-Genome Duplication. PLoS Genet 5(7): e1000549. https://doi.org/10.1371/journal.pgen.1000549
- ↑ 4.0 4.1 Prabhu, R.M., & Patel, R. (2004). Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis, and treatment. Clinical Microbiology and Infection, 10(1), 31-47
- ↑ Cox, G., Kauffman, C., & Thorner, A. (2019). Mucormycosis (zygomycosis). Uptodate
- ↑ About Mucormycosis, CDC. https://www.cdc.gov/fungal/diseases/mucormycosis/definition.html#:~:text=Rhinocerebral%20(sinus%20and%20brain)%20mucormycosis,have%20had%20a%20kidney%20transplant. Page last reviewed: August 9, 2021. Content source: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED)
- ↑ 7.0 7.1 7.2 Ibrahim, A. S., Spellberg, B., Walsh, T. J., & Kontoyiannis, D. P. (2012). Pathogenesis of mucormycosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 54 Suppl 1(Suppl 1), S16-22
- ↑ 8.0 8.1 8.2 Bhansali A, Bhadada S, Sharma A, et al.Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgraduate Medical Journal 2004;80:670-674
- ↑ 9.0 9.1 9.2 Lee BL1, Holland GN, Glasgow BJ Chiasmal infarction and sudden blindness caused by mucormycosis in AIDS and diabetes mellitus. Am J Ophthalmol. 1996 Dec;122(6):895-6
- ↑ Lam SC, Yuen HKL. Management of bilateral rhino-orbital cerebral mucormycosis. Hong Kong Med J. 2019;25(5):408-409. doi:10.12809/hkmj187588
- ↑ 11.0 11.1 Deb AK. Rhino-orbito-cerebral mucormycosis: etiopathology. Community Eye Health. 2021;34(113):7–9.
- ↑ 12.0 12.1 Chandran V, et al. Rhino-orbital mucormycosis presenting as orbital apex syndrome. Indian J Ophthalmol. 2021;69(5):1239–1242.
- ↑ Jiang N, Zhao G, Yang S, et al. A retrospective analysis of eleven cases of invasive rhino-orbito-cerebral mucormycosis presented with orbital apex syndrome initially. BMC Ophthalmol. 2016;16:10. Published 2016 Jan 12. doi:10.1186/s12886-016-0189-1
- ↑ Qingli, L., Orcutt, J., Seifter, L. (1989). Orbital mucormycosis with retinal and ciliary artery occlusions. British Journal of Ophthalmology, 73, 680-683
- ↑ Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-7115,6
- ↑ Lam Choi, V. B., Yuen, H. K., Biswas, J., & Yanoff, M. (2011). Update in pathological diagnosis of orbital infections and inflammations. Middle East African journal of ophthalmology, 18(4), 268-76.
- ↑ Herrera DA, Dublin AB, Ormsby EL, Aminpour S, Howell LP. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2009;19(2):117-125. doi:10.1055/s-0028-1096209
- ↑ Fahrenkopf MP, Nelson JJ, Eichhorn M, Conway J, Hassan A. Rhino-Orbital-Cerebral Mucormycosis and Orbital Exenteration. Eplasty. 2016;16:ic42. Published 2016 Nov 2.
- ↑ Raab P, Sedlacek L, Buchholz S, Stolle S, Lanfermann H. Imaging Patterns of Rhino-Orbital-Cerebral Mucormycosis in Immunocompromised Patients: When to Suspect Complicated Mucormycosis. Clin Neuroradiol. 2017;27(4):469-475. doi:10.1007/s00062-017-0629-1
- ↑ Skiada, A., Lanternier, F., Groll, A. H., Pagano, L., Zimmerli, S., Herbrecht, R., Lortholary, O., Petrikkos, G. L.(2013). Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica, 98(4), 492-504
- ↑ Rogers, T. (2008). Treatment of zygomycosis: current and new options. Journal of Antimicrobial Chemotherapy, 61(1), i35-i40
- ↑ Kauh CY, Nelson CC. Ophthalmic Pearls: Diagnosis and Management of Orbital MucormycosisEyeNet Magazine Jun 2014
- ↑ Shah K, Dave V, Bradoo R, Shinde C, Prathibha M. Orbital Exenteration in Rhino-Orbito-Cerebral Mucormycosis: A Prospective Analytical Study with Scoring System. Indian J Otolaryngol Head Neck Surg. 2019 Jun;71(2):259-265. doi: 10.1007/s12070-018-1293-8. Epub 2018 Mar 13. PMID: 31275841; PMCID: PMC6582081.
- ↑ Rapidis, A.D. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: Perspectives from a maxillofacial surgeon. Clinical Microbiology and Infection, 15(5), 98-102. DOI: https://doi.org/10.1111/j.1469-0691.2009.02989.x
- ↑ Vaughan, C., Bartolo, A., Vallabh, N., Leong, S.C. (2018). A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years?. Clinical Otolaryngology, 43(6), 1454-1464