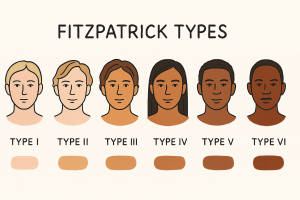
The Fitzpatrick Scale
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
Human skin tone is shaped by genetics, environment, and culture, and it exists on a continuum that resists simple categorization in clinical practice. Medical specialties such as dermatology and ophthalmology require classification methods as photobiology has been shown to inform risk, counseling, and periocular procedure planning. The Fitzpatrick Skin Type (FST) system, formalized by Fitzpatrick in 1975 and detailed in 1988, remains the most recognizable shorthand for cutaneous ultraviolet (UV) reactivity [1]. It was created to help clinicians select safe initial UVA doses for oral methoxsalen photochemotherapy (PUVA) in psoriasis[1] [2].
FST’s simplicity has resulted in broad adaptation (cancer risk counseling, phototherapy dosing, device selection), yet its self reporting origins make it vulnerable to misclassification, especially in diverse populations [3]. To bolster reproducibility and inclusivity, many centers now pair FST with objective color measurements such as CIELAB colorimetry and the Individual Typology Angle (ITA°), or with spectrophotometric indices that better capture constitutive pigmentation instead of a categorical, questionnaire based scale [4] [5].
The purpose of this article is to provide a comprehensive overview of the Fitzpatrick scale. It will include its historical evolution, describe its classification system, and explore its applications and limitations. Additionally, it will review some of the alternative classification systems since developed.
Historical Background
Thomas B. Fitzpatrick was an American dermatologist who served as Chair of Dermatology at Harvard Medical School and Chief of Dermatology at Massachusetts General Hospital from 1959-1987 [6]. He proposed “sun reactive skin typing” in 1975 to solve a pragmatic problem in PUVA: patients who looked similar (by hair/eye color) often did not tolerate the same UVA dose [1]. The initial categorization included lighter skin tones and only included types I-IV. Early PUVA reports established efficacy for oral methoxsalen and UVA in psoriasis and anchored the need for a dose-selection heuristic [2]. Over time, the four initial types were extended to a six-type scheme (I–VI) [1]. The system’s portability and shared vocabulary spurred global uptake, even as its white cohort origin and self-reporting core were recognized as limitations when applied broadly [1][3].
Classification
The Fitzpatrick Skin Scale is assigned by tendency to burn and ability to tan after sun exposure [1]. Type I “always burns/never tans”; Type VI “rarely or never burns/tans deeply,” with intermediate types in between. The scale estimates UV reactivity rather than constitutive skin color; its boundaries blur with mixed ancestry, variable sun histories, and patient recall.
| Fitzpatrick Type | Typical Skin Tone | Description |
|---|---|---|
| Type I | Very fair | Always burns, never tans; extremely sun-sensitive |
| Type II | Fair | Usually burns easily, tans minimally |
| Type III | Light to Medium | Sometimes burns, gradually tans to light brown |
| Type IV | Olive or Medium Brown | Rarely burns, tans easily to moderate brown |
| Type V | Brown | Very rarely burns, tans readily and deeply |
| Type VI | Deeply pigmented brown to dark brown | Almost never burns; deeply pigmented and sun-resistant |
Method of Assessment
Most clinicians still use a brief burn/tan questionnaire supplemented by phenotype cues (hair/eye/skin). The questionnaire format has the benefit of speed, however, although fast, self- report introduces recall bias and inter-observer variability, particularly for FST III–VI [3]. When dosing decisions or trial stratification matter, some centers add minimal erythema dose (MED) testing, which refers to the lowest dose of ultraviolet (UV) radiation that produces clearly perceptible redness on the skin within 24 hours of exposure, to anchor the subjective call [7][8] [9].
Objective Skin Tone / Phototype Measurement
Instrumental approaches complement FST and improve reproducibility. These methods are usually most helpful for darker phenotypes, in clinical trials, medicolegal documentation, and periocular energy-based procedures where pigmentation gradients matter:
- Colorimetry (CIELAB) and ITA°. CIELAB provides device independent L*a*b* values; the ITA° (computed from L* and b*) maps skin tone to continuous categories and tracks tanning kinetics ⁴,⁵. ITA° correlates with constitutive pigmentation but is not interchangeable with FST’s sun reactivity categories [10].
- Spectrophotometry /melanometry. objectively quantifies skin pigmentation by measuring light reflectance or melanin index at specific wavelengths, and when combined with the Fitzpatrick scale, it provides a more precise, reproducible assessment of both intrinsic pigmentation and UV reactivity [11].
Relevance to Ophthalmology
Phototype in ophthalmology is relevant whenever light becomes a tool, or a hazard, near the eye. Some applications include:
1) Periocular oncology & counseling. Fair phototypes carry a disproportionate burden of UV driven skin cancers; early counseling and vigilant surveillance are warranted in the periocular region [12].
2) Pigmentation traits and ocular disease (associations). Population studies have linked lighter irides with higher AMD prevalence, while dark brown irides show higher cataract risk in some cohorts [13] [14] [15]. It is important to note that FST does not map directly to ocular melanin, but pigmentation traits clearly intersect with ophthalmic disease.
3) Light based periocular procedures.
• Intense Pulsed Light (IPL) for dry eye due to MGD. In the United States, the FDA De Novo order (DEN200028) authorizes specific IPL devices for improvement of signs of dry eye due to MGD in adults with FST I–IV; application is limited to the malar region with ocular protection [16] [17]. Randomized trials demonstrate symptomatic and sign improvements, particularly when paired with meibomian gland expression [18] [19] [20].
• Periocular lasers/energy devices (resurfacing, pigment/vascular targets, hair reduction). Darker phototypes carry higher post inflammatory hyperpigmentation risk; wavelength choice and conservative parameters matter (e.g., long pulsed 1064 nm Nd:YAG for hair reduction in darker skin) [21] Device related ocular injuries are preventable with shields, strict technique, and adherence to device labeling [22].
Practical ophthalmic pearls
• Document FST and an objective measure (e.g., ITA° or melanin index) before periocular light based therapy.
• Use internal eye shields where indicated; avoid lash overlap with IPL; consider test spots in FST V–VI for any cosmetic periocular energy device.
• Clarify on label vs off label use when FST restrictions exist (e.g., IPL for DED in the U.S. is limited to FST I–IV) [16] [17].
Applications Beyond Ophthalmology
In dermatology, Fitzpatrick skin phototype (FST) continues to guide decisions where light is the therapeutic agent. Phototherapy algorithms for inflammatory dermatoses commonly allow initial NB‑UVB or PUVA dosing to be set by FST (or by a measured minimal erythema dose), with subsequent titration to clinical response, an approach that balances practicality with safety in routine care [23]. Energy‑based procedures likewise reference phototype when selecting wavelength, fluence, pulse duration, and cooling, reflecting the principles of selective photothermolysis and the higher risk of epidermal injury and post‑inflammatory hyperpigmentation as melanin optical density increases [24]. Outside medical treatment, objective color measurement has become central to cosmetic research and product development. Spectrophotometry and colorimetry in CIE L*a*b* space, often summarized by the Individual Typology Angle (ITA°), provide continuous, reproducible quantification of constitutive skin color across diverse populations and are widely used for shade design, product testing, and standardized reporting in clinical studies [4] [5]. In these contexts, FST serves as a complementary descriptor of sun reactivity, while instrumental color metrics supply the granularity needed for cross‑study comparability and inclusive formulation research [4] [5].
Limitations and Controversies
• Subjectivity and calibration. Self report and even visual clinician assignment can misclassify patients, with the largest errors in darker phototypes.
• Conflation of color and reactivity. FST started as a UV reactivity tool for white skin in PUVA and was later generalized; it is a poor proxy for constitutive color in global populations.
• Predictive shortcomings. Objective colorimetry/spectrophotometry often outperforms FST for skin cancer risk and for grouping together research cohorts.
Alternatives/Adjuncts and Recent Modifications
• CIELAB colorimetry / ITA° (Individual Typology Angle).
Objective skin color measurement using the CIE L*a*b* space, often summarized by ITA°, offers continuous, reproducible quantification of constitutive pigmentation. The method is fast and widely studied, maps tones into physiologic categories, and correlates strongly with spectrophotometric melanin indices; however, ITA° is not interchangeable with Fitzpatrick (which captures sun reactivity), so it serves best as an adjunct when decisions hinge on phototoxicity risk [10]
• Roberts Skin Type Classification System (RSTCS).
A 4 element, serial profile—phototype, hyperpigmentation tendency, photoaging, and scarring propensity—designed to predict response to injury/inflammation and to anticipate post inflammatory hyperpigmentation and scarring, particularly relevant when planning lasers/peels and other cosmetic procedures in diverse skin types. The author’s decennial update supports implementation and communication benefits in everyday practice [25]
• Taylor Hyperpigmentation Scale (THS).
A 15 shade visual tool that grades hyperpigmentation severity across FST I–VI; inexpensive, portable, and useful for baseline documentation and treatment response tracking in pigmentary disorders and trials [26]
• Representation scales for informatics (e.g., Monk Skin Tone [MST]).
A 10 tone scale introduced to improve dataset auditing and fairness evaluations in computer vision and related informatics. It is helpful for representation analysis but is not a clinical phototoxicity or treatment parameter tool; dermatology consensus pieces treat MST as complementary metadata rather than a replacement for physiologic or photobiologic measures [27]
• Proposed updates to Fitzpatrick type frameworks.
The Skin Color and Ethnicity (SCE) scale subdivides traditional FST IV–V into “A/B” categories to better stratify diverse tones for procedural and product reporting; this is a proposal with emerging uptake and requires broader validation before routine clinical substitution [28]
Summary
The Fitzpatrick Skin Phototype (FST) scale is a clinical shorthand that classifies skin by sun reactivity, or the tendency to burn or tan after ultraviolet exposure. It was originally introduced to guide safe dosing for PUVA photochemotherapy and later expanded to six categories (I–VI). Since its introduction in 1975, the FST scale remains useful across medicine because it is fast, familiar, and correlates with day to day decisions about photoprotection, device safety, and risk counseling. In ophthalmology, FST is relevant wherever light is a tool or a hazard near the eye: it helps frame periocular oncology counseling in fair phototypes; informs parameter selection and safety practices for periocular lasers and intense pulsed light; and aligns with device labeling (e.g., IPL for dry eye in the United States is indicated for adults with FST I–IV, with strict ocular protection). At the same time, FST has important limitations. It is subjective and historically Eurocentric (initially derived in lighter skinned cohorts), offers limited granularity for darker complexions, and conflates color with reactivity, two traits that only partially overlap. Because ocular melanin does not map directly to cutaneous phototype, pigmentation traits (such as iris color) should be considered separately when thinking about macular or lenticular disease. Modern practice therefore pairs the practical language of FST with objective measures, spectrophotometry/melanin index and colorimetry in CIE L*a*b*/ITA°, to add precision and inclusivity in research and clinical reporting. Adjacent frameworks such as the Roberts Skin Type Classification (capturing hyperpigmentation and scarring propensity) and the Taylor Hyperpigmentation Scale (grading pigment severity) can further refine risk assessment for procedures.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Fitzpatrick TB. The validity and practicality of sun reactive skin types I through VI. Arch Dermatol.1988;124(6):869 871.
- ↑ 2.0 2.1 Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974;291(23):1207 1211.
- ↑ 3.0 3.1 3.2 Eilers S, Bach DQ, Gaber R, et al. Accuracy of self report in assessing Fitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149(11):1289 1294.
- ↑ 4.0 4.1 4.2 Chardon A, Cretois I, Hourseau C. Skin colour typology and suntanning pathways. Int J Cosmet Sci.1991;13(4):191‑208.
- ↑ 5.0 5.1 5.2 Ly BCK, Dyer EB, Feig JL, Chien AL, Del Bino S. Research Techniques Made Simple: Cutaneous colorimetry. J Invest Dermatol. 2020;140(1):3 12.e1.
- ↑ Parrish JA. Thomas B. Fitzpatrick, MD, PhD (1919–2003). Arch Dermatol. 2003;139(12):1613.
- ↑ Conant L, Beck KM, Liao W. A rapid and cost effective device for testing minimal erythema dose. Dermatol Ther (Heidelb). 2018;8(3):483 489.
- ↑ British Association of Dermatologists & British Photodermatology Group. BAD/BPG Guidelines for Narrowband UV B Phototherapy. 2022. (Recommends MED/testing a small area for safe starting dose.)
- ↑ Heckman CJ, Chandler R, Kloss JD, Benson A. Minimal erythema dose (MED) testing: a practical overview. J Vis Exp. 2013;(75):e50175.
- ↑ 10.0 10.1 Osto M, et al. Individual Typology Angle and Fitzpatrick Skin Phototypes Are Not Interchangeable: A cross sectional analysis. Skin Res Technol. 2022;28(4):618 626. (Representative analysis showing ITA ≠ FST.)
- ↑ Vasudevan S, Vogt WC, Weininger S, Pfefer TJ. Melanometry for objective evaluation of skin pigmentation in pulse oximetry studies. Commun Med.
- ↑ Garbe C, Forsea A M, Amaral T, et al. Skin cancers are the most frequent cancers in fair skinned populations, but we can prevent them. Eur J Cancer. 2024;204:114074.
- ↑ Mitchell P, Smith W, Wang JJ. Iris color, skin sun sensitivity, and age related maculopathy: The Blue Mountains Eye Study. Ophthalmology. 1998;105(1):135 140
- ↑ Frank RN, Puklin JE, Stock C, Canter LA. Race, iris color, and age related macular degeneration. Trans Am Ophthalmol Soc. 2000;98:109 117.
- ↑ Cumming RG, Mitchell P, Lim R. Iris color and cataract: The Blue Mountains Eye Study. Am J Ophthalmol.2000;130(2):237 238.
- ↑ 16.0 16.1 U.S. Food and Drug Administration. De Novo Classification Order – DEN200028 (Lumenis Stellar M22). February 23, 2021. (Indication: improvement of signs of DED due to MGD in adults with FST I–IV; malar only; eye protection.)
- ↑ 17.0 17.1 Federal Register. Medical Devices; Ophthalmic Devices; Classification of the Intense Pulsed Light Device for Managing Dry Eye. Jan 20, 2023. (Class II; special controls; 21 CFR 886.5201.)
- ↑ Xue AL, Wang MTM, Ormonde SE, Craig JP. Randomised double masked placebo controlled trial of the cumulative treatment efficacy of IPL for MGD. Ocul Surf. 2020;18(2):286 297.
- ↑ Yan X, Hong J, Jin X, et al. The efficacy of intense pulsed light combined with meibomian gland expression for DED due to MGD: multicenter RCT. Eye Contact Lens. 2021;47(1):45 53.
- ↑ Ribeiro BB, Cruzat A, et al. Pulsed light therapy in dry eye management: a review. Clin Ophthalmol.2022;16:3613 3628.
- ↑ Quiñonez RL, Alexis AF, Dunnick CA, et al. An update on cosmetic procedures in people of color—Part 2 (includes laser hair reduction). J Am Acad Dermatol. 2022;86(4):729 739.
- ↑ Bonińska K, Bonikowski A, Żaba R, et al. Dermatologic laser induced ocular and periocular complications: a review. BMC Ophthalmol. 2023;23:311.
- ↑ Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81(3):775‑804.
- ↑ Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220(4596):524‑527.
- ↑ Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7(5):448 451.
- ↑ Taylor SC, Arsonnaud S, Czernielewski J. The Taylor Hyperpigmentation Scale. Cutis. 2005;76(4):270 274.
- ↑ Monk EP, Monk Skin Tone Scale (preprint/overview). SocArXiv. 2022. (Representation/fairness scale; not a clinical phototoxicity tool.)
- ↑ Coleman W, Mariwalla K, Grimes P. Updating the Fitzpatrick Classification: The Skin Color and Ethnicity Scale. Dermatol Surg. 2023;49(8):725 731. doi:10.1097/DSS.0000000000003860.