Torpedo Maculopathy
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
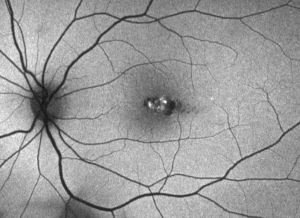
Torpedo maculopathy (TM), also known as solitary hypopigmented nevus of the retinal pigment epithelium (RPE), paramacular albinotic spot syndrome, congenital hypomelanotic freckle, or atypical macular coloboma was first described by Roseman and Gass in 1992 as a rare congenital anomaly of the RPE that produces a disruption of outer retinal layers [1]. So far, short series and scarcely any data about prevalence, demographics or incidence have been reported. Pathogenesis remains unknown and the typical lesion is a single hypopigmented area in the macula, asymptomatic, temporal to fovea and with a characteristic torpedo-shape. No familial cases of TM have been reported.[2]
Disease Entity
Epidemiology
Shirley et al. published an eight-case series showing a slightly female predominance with a mean age at diagnosis of 8 years old (range 3-15). Based on this study, the prevalence was estimated to be 2/100,000 in subjects less than 16 years old who reside in Northern Ireland (Belfast), which is probably an underestimate given it is an asymptomatic condition [3].
Pathophysiology
Several theories have been proposed to explain the pathogenesis of TM:
- A developmental deficiency in the nerve fiber layer along the horizontal raphe, [4] with nasal aspects of the lesions localized to a kite-shaped region of low nerve fiber layer density. [5]
- Abnormal choroidal circulation or ciliary vasculature development [6][7]
- Persistent developmental defect in the RPE of the fetal temporal bulge, which is an aggregation of these cells occurring between 4 and 6 months of gestation. It is usually found at 4mm distance from the optic nerve, temporal to the fovea, progressively decreasing between 6 and 8.5 gestational months. There is therefore speculation about a congenital RPE defect at a given point in fetal development [8].
- A consequence of intrauterine chorioretinitis [9]
Diagnosis
Manifestations
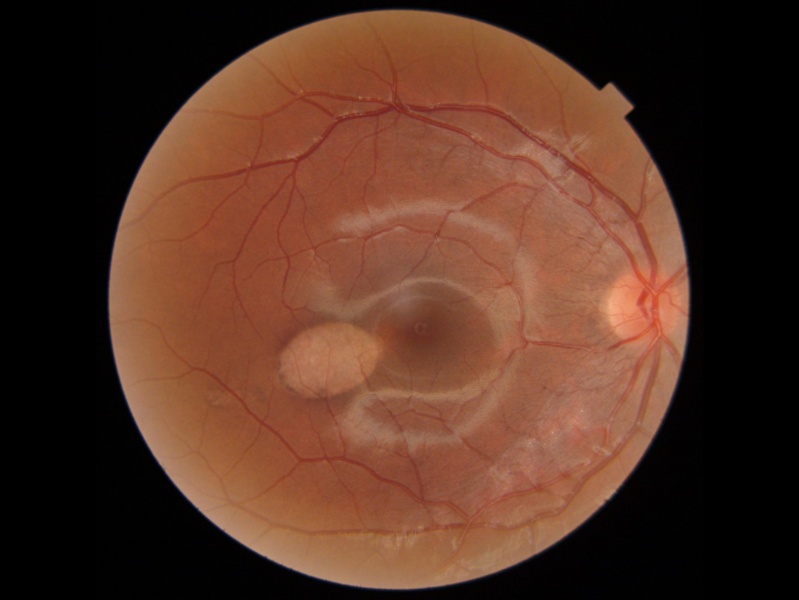
On exam, visual acuity is not generally affected since there is no foveal involvement and the lesion is usually an incidental finding. Nevertheless, central scotoma or microscotoma may be present at diagnosis. It is typically unilateral, flat, asymptomatic and non-progressive, although bilateral, crescent and satellite lesions have also been reported [3] [10] [11][12]. Furthermore, an association with neurosensory retinal detachment or multifocal central serous chorioretinopathy has been published [13][14] as well as two cases with a double-torpedo lesion [15][16]. Only 8 cases have been reported in the literature of choroidal neovascularization (CNV) associated with TM, as it is typically stable [3][17] [18][19][20][21][22][23]. Six of these cases contained a choroidal excavation, which has been associated with CNV and could be the explanation over a complication of TM. [24] Owing to the RPE and outer retina damage, these lesions are susceptible to CNV development and need to be monitored[3]. There is a characteristic hypopigmented torpedo-shaped area temporal to the macula, which points to the fovea with a hyperpigmented tail. However, rare cases of globally hyperpigmented TM have been described in the literature,[25][26][27] and Jain et. al reported 1 case of TM on the inferonasal side of the disc[28]. Regardless, the typical size is about two disc diameters horizontally by one disc diameter vertically, usually without foveal involvement [29].
Associations
Tsang et al.[11] have theorized that TM may be associated with other systemic disorders such as kidney disease, blepharophimosis, situs inversus, choroidal nevi, and ametropias. Packo and Goldberg[30] report an association between fundus abnormalities in Gardner Syndrome and TM and recommend genetic testing for individuals with classic torpedo lesions. Hansen et al.[31] described TM in association with tuberous sclerosis and astrocytic hamartoma. Manesh et al.[32] described a case of unilateral TM in coexistence with bilateral retinal flecks. Turkoglu et al. report a case of TM with a coexistence of retinoblastoma but found no relationship through genetic analysis.[33] Alarcon-Martinez et. al report a case of TM associated with a novel de novo NEXMIF mutation, an X chromosome gene that may play a role in eye development.[34] However, Celik et al. conducted a retrospective review of 17 infants diagnosed with TM and concluded that no pathogenic variant of NEXMIF gene was observed.[35] In addition, Shields et al.[8] maintained that there is no clear evidence of any systemic association.
Differential diagnosis [36][9]
| Condition | Entity |
|---|---|
| Old retinal lesions | Chorioretinal scar |
| Trauma | Retinal trauma |
| Congenital lesions | Congenital RPE hypertrophy
Gardner syndrome-associated congenital RPE hypertrophy Congenital RPE albinotic spots |
| Tumors | RPE hamartoma
Combined RPE-retinal hamartoma Benign melanoma or acquired |
| Retinal dystrophies | Viteliform dystrophy or pattern dystrophies |
Clinical diagnosis
The pathognomonic lesion on funduscopic examination is solitary, hypopigmented, oval-shaped with hints of a bullet or a torpedo and a wedge-shaped tail extending peripherally and pointing toward the foveolar region along the horizontal raphe, although rare cases may be outside of the horizontal raphe but oriented along the nerve fiber layer path with the nasal apex pointing to the optic disc.[37]
Diagnostic procedures
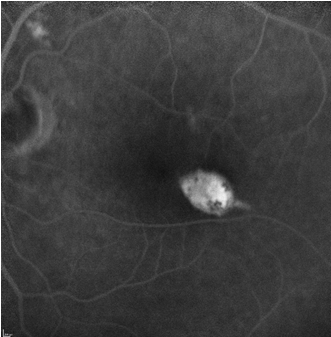
- Fundus Autofluorescence: Hyperfluorescent border (composed of lipofuscin produced by dysfunctional RPE cells or those under metabolic stress) surrounding the hypofluorescent area (atrophic or dead RPE), depending on the size involved [40].
- Fluorescein Angiography: Transmission hyperfluorescence (window effect) due to atrophy of the retinal pigment epithelium without leakage [41]
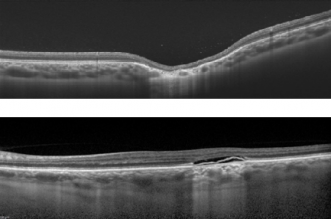
- Optical Coherence Tomography (OCT): In 2015, Wong et al. [42] proposed a classification of TM into two types according to the pattern of abnormality on OCT. In both types, there is attenuation of outer retinal structures (explained by RPE thinning and photoreceptor loss that can be attributed to several developmental defects [10] [11]) with an increased signal transmission along the choroid and conservation of inner layers.
- Type 1 include lesions that show attenuation of interdigitation zone and ellipsoid zone without outer retinal cavitation.
- Type 2 show loss of ellipsoid and interdigitation zones as well as thinning of the outer nuclear layer associated with outer retinal cavitation. Inner choroidal excavation may be present or absent[42][43].
- Type 3 lesions have been suggested as an additional category defined by excavated inner layers, retinal thinning, inner retinal hyper-reflective spaces, and no subretinal cleft [43]. Three cases of type 3 lesions have reported the presence of subretinal fluid.[44]
- Wong et al.[42] postulated Type 1 lesions evolve into Type 2 ones over several decades, although this has been refuted by Shirley et al.[3], asserting that the difference is phenotypical rather than temporal[42]. Rickmann et al. also found that there was no age difference between patients with Type 1 and Type 2 lesions, and type 2 TM can also occur in young patients.[45]
- Light and Liu proposed a type 4 morphology, reporting a case in which the foveal portion showed disruption of the ellipsoid zone without outer retinal cavitation, while the temporal tail had a preservation of the ellipsoid zone and inner choroidal excavation. [46]
- OCT Angiography (OCTA): a recent non-invasive tool to study retinal and choroidal vasculature. The primary site of malformation lies in the RPE/choriocapillaris complex [47][48][49][50]. There is a normal superficial retinal plexus and attenuation of signal in the deep vascular layers along the lesion, with loss of deep vessels in the subretinal gap. Variations in deep retinal layers develop with time and are not in the location of the lesion. Other findings range from diffuse attenuation of the choriocapillaris, hyporeflectivity due to atrophy correlating to the OCT subretinal gap and adjacent disruption near the tail [51]. Choroidal vessels remain unaffected. OCTA en face imaging shows decreased flow in the area of the subretinal cleft associated with hyper-reflectivity in the tail area[48].
- Microperimetry: Reduction of retinal sensitivity along the torpedo lesion which remains stable during follow-up. Seldom, sensitivity may worsen over time owing to persistent alterations of outer retinal layers and choriocapillaris[47].
- Retromode Imaging (RMI): Lopez et al. reported on the use of RMI to generate a simulated pseudo-3-dimensional image for TM. This non-invasive technique is effective at visualizing retinal structures and confirming the presence of subretinal fluid under the neurosensory retina.[52]
- ·Multicolor imaging (MC): Venkatesh et al. reported on the use of multicolor imaging with TM. Blue wavelengths captured superficial retinal structures, green wavelengths captured vascular structures, and infrared wavelengths displayed the retinal pigment epithelium and choroid. MC imaging can assist in defining retinal and choroidal involvement of TM.[53]
Management
In general, TM is a congenital condition that remains stable over years with minimal risk of vision loss. Trevino et al. conducted one of the longest reported follow-ups of 2 patients with TM, and no clinically significant progression occurred over the ten year period, with lesion morphology and visual function remaining stable.[54] Nevertheless, due to the rare possibility of CNV or progression, periodic monitoring of these patients has been recommended[13]. The pathophysiology of TM remains unclear on whether it is static and or has a progressive development of fluid accumulation.[55]
References
- ↑ Roseman RL, Gass JD. Solitary hypopigmented nevus of the retinal pigment epithelium in the macula. Arch Ophthalmol. 1992; 110:1762.
- ↑ Liu, Y., Moore, A.T. Congenital focal abnormalities of the retina and retinal pigment epithelium. Eye 34, 1973–1988 (2020). https://doi.org/10.1038/s41433-020-0902-4
- ↑ 3.0 3.1 3.2 3.3 3.4 Shirley K, O’Neill M, Gamble R, Ramsey A, McLoone E. Torpedo maculopathy: disease spectrum and associated choroidal neovascularization in a paediatric population. Eye. 2018; 32:1315-20.
- ↑ Pian D, Ferrucci S, Anderson SF, Wu C. Paramacular coloboma. Optom Vis Sci. 2003; 80:556-63.
- ↑ Williams PJ, Salek S, Prinzi RA, Bergstrom C, Hubbard GB. Distribution patterns of torpedo maculopathy: Further evidence of a congenital retinal nerve fiber layer-driven etiology. Saudi Journal of Ophthalmology. 2019;33(3):260-267. doi:10.1016/j.sjopt.2019.07.010
- ↑ Teitelbaum BA, Hachey DL, Messner LV. Torpedo maculopathy. J Am Optom Assoc. 1997; 68:373-6.
- ↑ Papastefanou VP, Vázquez-Alfageme C, Pearse AK et al. Multimodality imaging of Torpedo maculopathy with swept-source, en face optical coherence tomography and optical coherence tomography angiography. Retin Cases Brief Rep. 2016 Oct 19 ([Epub ahead of print]).
- ↑ 8.0 8.1 Shields CL, Guzman JM, Shapiro MJ, Fogel LE, Shields JA. Torpedo maculopathy at the site of the fetal bulge. Arch Ophthalmol. 2010; 128:499-501.
- ↑ 9.0 9.1 De Manuel-Triantafilo S, Gili P, Bañuelos J. Torpedo maculopathy: two case reports and a literature review. Arch Soc Esp Oftalmol. 2016; 91(8):400-3.
- ↑ 10.0 10.1 Sanabria MR, Coco RM, Sanchidrian M. OCT findings in torpedo maculopathy. Retin Cases Brief Rep. 2008; 2:109-11.
- ↑ 11.0 11.1 11.2 Tsang T, Messner LV, Pinol A et al. Torpedo maculopathy: In-vivo histology using optical coherence tomography. Optom vis sci. 2009;86: E1380-85.
- ↑ Richez F, Gueudry J, Brasseur G, Muraine M. Bilateral torpedo maculopathy. J Fr Ophthalmol. 2010; 33(4): 296.
- ↑ 13.0 13.1 Su Y, Gurwood AS. Neurosensory retinal detachment secondary to torpedo maculopathy. Optometry. 2010; 81: 405-7.
- ↑ Panigrahi PK, Minj A, Satapathy J.Torpedo maculopathy with multifocal central serous chorioretinopathy: A rare case report. Indian J Ophthalmol. 2018; 66(2):330-1.
- ↑ Raju B, Nooyi C, Raju NSD, Nidheesh S. Torpedo maculopathy with double torpedoes. Indian J Ophthalmol. 2018; 66(8):1189-90.
- ↑ Hussain F, Kasetty VM, Hamad A. Double torpedo maculopathy in an adolescent patient. Indian Journal of Ophthalmology - Case Reports. 2022;2(3):725. doi:10.4103/ijo.IJO_3060_21
- ↑ Agarwal A. Gass’ Atlas of macular diseases. 1076. Toronto, Canada: Elsevier Health Sciences; 2011.
- ↑ Jurjevic D, Boni C, Barthelmes D, et al. Torpedo maculopathy associated with choroidal neovascularization. Klin Mon Augenheilkd. 2017; 234: 508-14.
- ↑ Parodi MB, Romano F, Montagna M, Albertini GC, Pierro L, Arrigo A, Bandello F. Choroidal neovascularization in Torpedo Maculopathy Assessed on Optical Coherence Tomography Angiography. Ophthalmic surg lasers Imaging Retina. 2018; 49(11): e210-13.
- ↑ Banaee T, Doss M, Eller AW. Torpedo maculopathy complicated by choroidal neovascularization. Am J Ophthalmol Case Rep. 2020;19:100772. doi:10.1016/j.ajoc.2020.100772
- ↑ Byer M, Rousso L, Rodman J, Shechtman D. Case Report: Use of Multimodal Imaging to Document a Rare Complication of Torpedo Maculopathy. Optom Vis Sci. 2021;98(8):870-875. doi:10.1097/OPX.0000000000001748
- ↑ Ghezzaz A, Idlefqih W, Chahed S, Mahdjoubi A. CHOROIDAL NEOVASCULARIZATION IN TORPEDO MACULOPATHY TREATED BY AFLIBERCEPT: LONG-TERM FOLLOW-UP USING OPTICAL COHERENCE TOMOGRAPHY AND OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY. Retin Cases Brief Rep. 2023;17(4):433-437. doi:10.1097/ICB.0000000000001213
- ↑ Soman M, Indurkar A, Mohan A, Nair U. Secondary choroidal neovascular membrane in a case of torpedo maculopathy. Indian J Ophthalmol. 2020;68(11):2499-2500. doi:10.4103/ijo.IJO_468_20
- ↑ Trevino RC, Ridder WH, Laul A, Hill J. Long-term follow-up of torpedo maculopathy: a case series and mini-review. BMC Ophthalmol. 2024;24(1):5. doi:10.1186/s12886-023-03254-z
- ↑ Rohl A, Vance S. Hyperpigmented Torpedo Maculopathy with Pseudo-Lacuna: A 5-Year Follow-Up. Case Rep Ophthalmol. 2016;7(1):184-190. doi:10.1159/000445497
- ↑ Ranjith PC, Giridhar A. Hyperpigmented torpedo maculopathy. Indian J Ophthalmol. 2019;67(2):270-271. doi:10.4103/ijo.IJO_886_18
- ↑ Chitturi SP, Venkatesh R, Handa A, Mangla R, Chhablani J. A rare case of hyperpigmented torpedo maculopathy. European Journal of Ophthalmology. 2024;34(1):NP108-NP110. doi:10.1177/11206721231182736
- ↑ Jain S, Kumawat D, Kumar V. Multimodal imaging of torpedo-shaped fundus lesions: New insights. Indian J Ophthalmol. 2018;66(8):1211-1213. doi:10.4103/ijo.IJO_118_18
- ↑ Trevino R, Kiani S, Raveendranathan P. The expanding clinical spectrum of torpedo maculopathy. Optom Vis Sci. 2014; 91: S71-8.
- ↑ Packo K, Goldberg MF. Torpedo-like lesions in the ocular fundi of Gardner syndrome: hiding in plain view. Ophthalmic Genet. 2021;42(5):514-520. doi:10.1080/13816810.2021.1925930
- ↑ Hansen MS, Larsen M, Hove MN. Optical coherence tomography of torpedo maculopathy in a patient with tuberous sclerosis. Acta Ophthalmol. 2016; 94(7): 736-737.
- ↑ Kumar M, Singh K, Ravichandra G, Patel A, Kapoor M, Bhargava R. Torpedo maculopathy with bilateral familial fleck retina: An unusual coexistence. Indian Journal of Ophthalmology - Case Reports. 2022;2(2):458. doi:10.4103/ijo.IJO_1610_21
- ↑ Turkoglu EB, Erol MK, Karaca BO. Coexistence of torpedo maculopathy and retinoblastoma: Differentiation the lesions with hand-held optical coherence tomography. Photodiagnosis and Photodynamic Therapy. 2021;34:102331. doi:10.1016/j.pdpdt.2021.102331
- ↑ Alarcon-Martinez T, Khan A, Myers KA. Torpedo Maculopathy Associated with NEXMIF Mutation. Molecular Syndromology. 2019;10(4):229-233. doi:10.1159/000498835
- ↑ Celik G, Gunay M, Vural A, Kizilay O, Demirkol YK, Erol MK. Clinical evaluation of torpedo maculopathy in an infant population with additional genetic testing for NEXMIF mutation. Eye. 2022;36(8):1639-1644. doi:10.1038/s41433-021-01714-8
- ↑ Golchet PR, Jampol LM, Mathura JR jr, Daily MJ. Torpedo maculopathy. Br J Ophthalmol. 2010;94: 302-6.
- ↑ Smith MJ, Sia DIT, Greve M. Torpedo maculopathy—inferior variant. Canadian Journal of Ophthalmology. 2024;59(1):e94-e96. doi:10.1016/j.jcjo.2021.02.008
- ↑ Photo courtesy of Jaume.Catala-Mora MD.
- ↑ 39.0 39.1 Photo courtesy of Ignacio.Flores-Moreno MD.
- ↑ Thomas AS, Flaxel CJ, Pennesi ME. Spectral-domain optical coherence tomography and fundus autofluorescence evaluation of torpedo maculopathy. J Pediatr Ophthalmol Strabismus. 2015; 52: e8-e10.
- ↑ Rigotti M, Babighian S, Carcereri De Prati E, Marchini G. Three cases of a rare congenital abnormality of the retinal pigment epithelium: torpedo maculopathy. Ophthalmologica. 2002;216(3):226-227. doi:10.1159/000059639
- ↑ 42.0 42.1 42.2 42.3 Wong E, Fraser-Bell S, Hunyor A, Chen F. Novel optical coherence tomography classification of torpedo maculopathy. Clin Exp Ophthalmol. 2015; 43:342-8.
- ↑ 43.0 43.1 Tripathy K, Sarma B, Mazumdar S. Commentary: Inner retinal excavation in torpedo maculopathy and proposed type 3 lesions in optical coherence tomography. Indian J Ophthalmol. 2018; 66(8):1213-4.
- ↑ Wang DJ, Mendel TA. A unique presentation of subretinal fluid in a type III torpedo maculopathy phenotype. American Journal of Ophthalmology Case Reports. 2024;33:101971. doi:10.1016/j.ajoc.2023.101971
- ↑ Rickmann A, Bodenbender JP, Gelisken F, Kühlewein L. Type 1 and type 2 torpedo maculopathy. Graefes Arch Clin Exp Ophthalmol. 2024;262(6):1805-1810. doi:10.1007/s00417-024-06386-0
- ↑ Light JG, Alvin Liu TY. A novel phenotype of torpedo maculopathy on spectral-domain optical coherence tomography. Am J Ophthalmol Case Rep. 2020;20:100956. doi:10.1016/j.ajoc.2020.100956
- ↑ 47.0 47.1 Grimaldi G, Scupola A, Sammarco MG, Marullo M, Blasi MA. Morpho-functional evaluation of torpedo maculopathy with optical coherence tomography angiography and microperimetry. Am J Ophthal Case Rep. 2018; 10:165-8.
- ↑ 48.0 48.1 Hamm C, Shechtman D, Reynolds S. A deeper look at torpedo maculopathy. Clin Exp Optom. 2017; 100: 563-8.
- ↑ Ali Z, Shields CL, Jasani K, Aslam TM, Balaskas K. Swept-source optical coherence tomography angiography findings in Torpedo maculopathy. Ophthalmic Surg Laser Imag Retina. 2017; 48(11):932-935.
- ↑ Giannakaki-Zimmermann H, Munk MR, Dysli C et al. Optical coherence tomography angiography features of Torpedo maculopathy. Retin Cases Brief Rep. 2017 Apr 3 ([Epub ahead of print]).
- ↑ Papastefanou VP, Vázquez-Alfageme C, Pearse AK, et al. Multimodality imaging of Torpedo maculopathy with swept-source, en face optical coherence tomography and optical coherence tomography angiography. Retin Cases Brief Rep. 2016 Oct 19 ([Epub ahead of print]).
- ↑ Lopez JM, Miere A, Rabinovic M, et al. Torpedo maculopathy: multimodal and retromodal imaging. Canadian Journal of Ophthalmology. 2024;59(1):e96-e99. doi:10.1016/j.jcjo.2023.10.004
- ↑ Venkatesh R, Bavaharan B, Yadav NK. Multicolor imaging findings in torpedo maculopathy. Indian J Ophthalmol. 2019;67(2):295-297. doi:10.4103/ijo.IJO_1317_18
- ↑ Trevino RC, Ridder WH, Laul A, Hill J. Long-term follow-up of torpedo maculopathy: a case series and mini-review. BMC Ophthalmol. 2024;24(1):5. doi:10.1186/s12886-023-03254-z
- ↑ Kerwat D, Jamall O, Antonakis S, Almeida GC. Torpedo maculopathy: A case series – insights into basic pathology. European Journal of Ophthalmology. 2021;31(3):NP35-NP39. doi:10.1177/1120672120905313Patients can self-monitor as well with an Amsler grid, although in cases of large lesions or the presence of pigment clumping, more frequent observation may be indicated.