Trematode Induced Uveitis
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Article summary goes here.
Disease Entity
Trematode induced uveitis is a collective name for different uveitic presentations affecting patients swimming in trematode infested waters of some developing countries.
Etiology
The disease is thought to be caused by various trematode species living in fresh waters with complex lifecycles that include one or more intermediate hosts including snails, fresh water fish and crustaceans with definite hosts being aquatic birds. Humans get infected as accidental hosts after coming in contact with contaminated waters containing cercaria released from trematode infected snails during bathing, swimming or working (Figure 1). Molecular analysis of surgically excised samples don’t always yield positive results yet fragments of DNA belonging to digenic trematodes, such as Procerovum varium, have been successfully identified in patients from India and Egypt.
Risk Factors
Although patients of any age can be affected, yet the disease seems to be more common among children and adolescents with serious visual sequelae in younger children due to more aggressive disease and late presentations with complications.
Pathophysiology
So far, the exact route of pathogen entry into the eye is unknown. Ocular involvement is thought to be due to direct penetration of ocular tissues by cercaria in infested waters which are known to penetrate skin and mucous membranes. Swimmers usually report generalized itching shortly after bathing in contaminated waters with swelling of their buccal mucosa. It is uncertain if hematogenous spread of eggs or cercarial antigens after swallowing of infested water or ingesting undercooked fish can be another possible mode of infection. Fecal analysis for trematode eggs in ocular patients is usually negative suggesting that this is unlikely.
Primary Prevention
Prevention is mainly through avoiding direct contact with contaminated waters especially in young age groups,
Diagnosis
History
Disease onset is closely related to prior swimming in shallow, slow-running fresh waters of tributaries of main rivers or brackish ponds. Patients usually start to show symptoms of generalised itching shortly after coming into contact with the water followed few days later by variable degrees of eye redness, pain and diminution of vision.
Physical Examination
Signs and symptoms depend on the site of granuloma formation with a general rule of a more serious disease the more posterior the affection. Granuloma(s) can form anywhere in the eye from the conjunctiva and episclera anteriorly to the retina and choroid posteriorly. In many cases, a single patient can have more than one granuloma in the ipsilateral eye or can have bilateral presentations with different granuloma sites in each eye.
Signs
1 Conjunctiva & Episclera
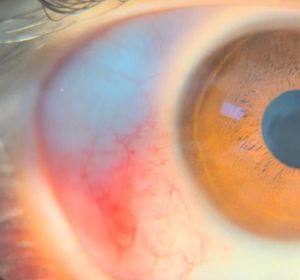
Usually children with conjunctival and episcleral affection present with redness and pain related to one or more nodules in the lower 180 degrees of one or both eyes. The nodule(s) tend to be small and well-circumscribed (Figure 2).
2.. Cornea
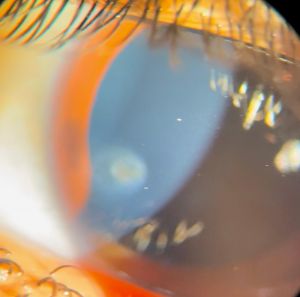
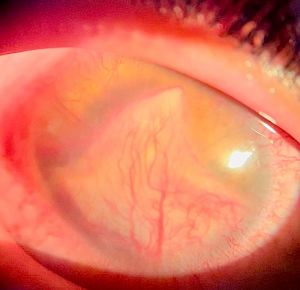
When parasitic antigens settle within the cornea through limbal blood vessels, they tend to form a yellowish, white granuloma commonly close to the limbus with surrounding localised area of stromal keratitis and neighboring ciliary injection (Figure 3). Extension of inflammation towards endothelium may lead to low grade anterior chamber inflammation with cells and flare. In most cases, the corneal lesion tends to heal with fibrosis likely after the use of topical steroids resulting in a greyish white fibrotic scar which might exhibit a yellow centre in some cases (Figure 4). The healed stromal lesion, being paracentral, may be asymptomatic or may present in the form of astigmatic error due to deformation of stromal fibres.
3. Anterior Chamber
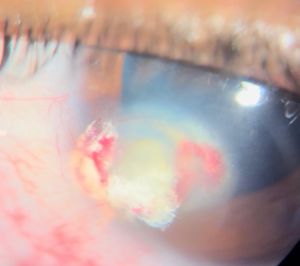
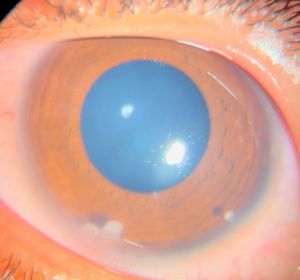
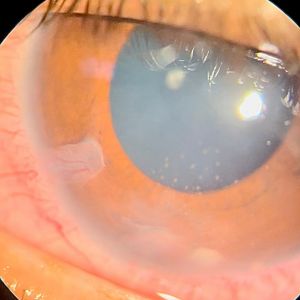
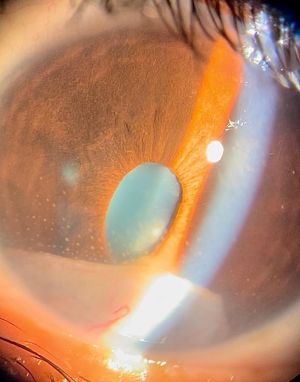
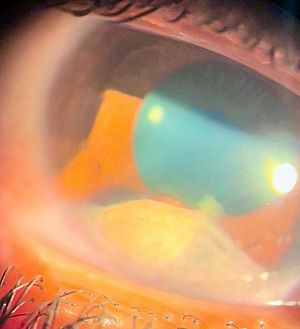
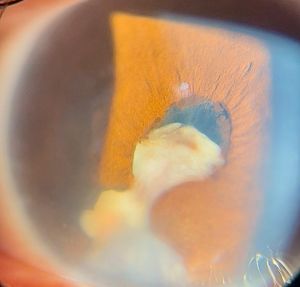
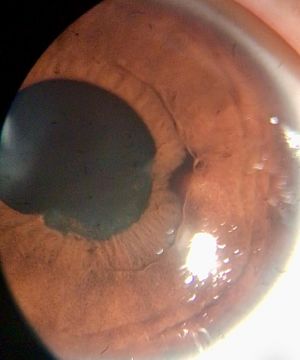
Anterior chamber (AC) granuloma is perhaps the most common and recognized presentation of the disease in which one or more pearly like, yellowish white nodules are present in the inferior angle; usually between 4 and 8 o'clock positions (Figure 5). The nodule excites an intense granulomatous anterior chamber reaction which might be associated with granulomatous keratic precipitates (KPs) on the back of the lower cornea close to the AC granuloma (Figure 6). In severe cases, vitritis can also occur even in the absence of any posterior granulomas. The granuloma(s) vary in size and can range from a small single nodule to large cheesy granulomas occupying several clock hours of the AC (Figure 7). Another kind of the AC granuloma is the membranous type which is characterized by the formation of a strong, adherent retrocorneal membrane with variable degrees of vascularisation especially in chronic cases (Figure 8). This membrane tends to form very strong adhesions with the neighboring iris resulting in peaking of the pupil with poor pupillary dilation; an important implication in patients requiring cataract or posterior segment surgeries later on. In some cases, there may be more than one AC granuloma with variable sizes and upper locations above the 3-9 o'clock horizontal meridian or a mixture of both types of granulomas: nodular and membranous (Figure 9). It is important in all cases of AC granuloma to perform gonioscopy to detect small microscopic granulomas that may be hidden anywhere in the angle. Unfortunately, in the setting of painful red eyes of active patients, this may prove to be very challenging. Posterior extension of an AC granuloma can occur leading to lenticular affection with localized cataract formation (Figure 10).
4. Iris
Granuloma formation within iris stroma results in the formation of a localized nodule with surrounding thickening of the iris resulting in a characteristic bulge. The presentation is usually associated with AC reaction and posterior synechiae close to the site of the nodule (Figures 11). Posterior protrusion of the granuloma beyond posterior surface of the iris may lead to lenticular spread resulting in cataract formation. Perhaps this is one of most difficult sites to treat due to difficult surgical excision and recurrence after tapering of steroids.
5. Lens
Lenticular granulomas usually occur as extensions from granulomas originally arising in the ciliary body (CB) posteriorly that have progressed anteriorly to the periphery of the lens as well as the iris root. In these cases, localized cataract close to the ciliary body granuloma (CBG) is quite characteristic with involvement of the posterior capsule of the lens by the granuloma (Figure 12). Anterior chamber and iris granulomas can also extend posteriorly into the lens as mentioned before. In other cases, lenticular granulomas can occur solely within the lens where they are usually found at the inferior equator with gradually progressive cataract (Figure 13).
6. Ciliary Body
Ciliary body granulomas are the least recognized forms of trematode induced uveitis inspite of being the most visually threatening entity. The granuloma forms within the ciliary body usually in the inferior quadrants (Figure 14) and excites significant inflammation in the form of vitritis (up to Grade IV) (Figure 15) and even retinal vasculitis which gave rise to the term "Presumed trematode induced granulomatous intermediate uveitis" (PTIGIU) proposed by Amin at al. When CBG is localized to one quadrant, vitritis tends to be most dense close to the lesion with vitreous bands pointing towards it (Figure 16). CBG can be as small as 1-2 clock hours or can be extensive with 360 degrees spread. Progressive enlargement of the granuloma with subsequent fibrosis can occur in any direction: 1. Anteriorly, it results in involvement of the iris root and periphery of the lens which might lead to localized cataract and significant lenticular astigmatism (Figure 17). Left untreated, the cataract can progress to total lens opacity (Figure 18). In these cases, a localized forward bulge of the iris at the corresponding clock hour of the underlying CBG acts as a clue to the presence of the latter (Figure 19). 2. If the CBG extends circumferentially (especially in young children with chronic disease), complications in the form of supraciliary effusion, cyclitic membranes, choroidal detachment and intractable hypotony may be present which pose the most difficult cases to treat with poor prognosis. 3. Posterior, radial extension of the granuloma towards the retina causes the peripheral retina to become dragged towards the granuloma (Figure 20). Subsequent contraction of the fibrous element of the granuloma results in tractional retinal detachment (TRD). Amin et al, through surgical treatment of these cases, divided these cases into early "active" phase where the retina was still attached at presentation and late "cicatricial" phase where patients developed TRD. In extreme cases, subretinal fibrotic bands extend from the CBG towards the disc aggravating the TRD present (Figure 21). Cases of CBG, are less common than AC granuloma cases, yet they pose higher risk of visual loss due to retinal involvement. Unfortunately, patients in the cicatricial phase are at risk for recurrent retinal detachment after initial pars plana vitrectomy with poor visual prognosis compared to patients presenting in the active phase. When left untreated, CBG eyes can pass into phthisis bulbi which is catastrophic considering that it can be bilateral in a young child.
7. Retina, Choroid and Optic Nerve
The most visually compromising trematode induced granulomas are those arising in the CBG with posterior extension resulting in TRD as explained above. In very rare cases, it was found that granulomas can occur within the choroid, especially at the posterior pole. These cases were associated with the concurrent presence of CBG and had elements of exudative retinal detachment secondary to the severe posterior inflammation. Even in the absence of choroidal granulomas, cases with CBG exhibit variable grades of retinal vasculitis, macular edema and hyperemic discs compared to fellow normal eyes. These findings tend to disappear with treatment. Follow-up of some of CBG cases after treatment showed that some patients may be at risk for developing epiretinal membranes with significant tangential traction on inner retinal layers.
8. Atypical cases
In very rare cases, trematode induced granulomatous uveitis was associated with acute ipsilateral dacryoadenitis which was significant in one case leading to mild dystopia (Figure 22). Excisional biopsy of the lacrimal gland was done in this case revealing non-specific chronic inflammatory reaction on histopathological analysis with resolution of the inflammation after a short course of systemic steroids. In another case, the ipsilateral lacrimal gland enlargement was associated with calcification visible on Computed Tomography (CT) of the orbit. It is unknown whether this was related to the associated ipsilateral CBG that was present in this case or not.
Symptoms
Symptoms vary according to age and site of granuloma formation. Patients tend to present shortly following contact with contaminated waters with acute, red, painful eyes that tend to respond well to topical steroids and have a tendancy to recur with tapering of the drops. Patients having large sized granulomas in the anterior chamber may notice the developing of a "white spot" corresponding to the AC granuloma and / or retrocorneal fibrous membrane. Depending on the severity of the inflammatory reaction and extent of the disease, patients can present with diminution of vision due to cataract, vitritis, macular edema and retinal detachment.
Diagnostic Procedures
Laboratory Tests
All patients presenting with presumed trematode induced uveitis should undergo laboratory workup and imaging to exclude other causes of granulomatous uveitis such as complete blood picture with differential, erythrocyte sedimentation rate, C-reactive protein, toxoplasmosis serology, toxocariasis serology and Quantiferon Gold Tb Test. Considering how Tuberculosis is also endemic in these developing countries, a positive Quantiferon Gold TB Test does not necessarily indicate tuberculous uveitis in these cases.
Ocular Investigations
Up til now, having a high index of clinical suspicion in a clinical setting compatible with trematode induced uveitis, especially in young patients with positive history of prior contact with brackish fresh water, is considered the golden standard for diagnosis of these cases. Diagnosis is usually straightforward in cases of characteristic AC granuloma and/ or retrocorneal membrane.
In cases of more posterior granulomas, ultrasound biomicroscopy (UBM) plays a crucial role in the confirmation of the disease and assessment of its extent. UBM performed by expert personnel can detect the presence of microscopic anterior chamber granulomas in addition to iris, lenticular and CB granulomas. Perhaps the most important role of UBM is to detect crucial findings related to CBG such as: degree of anterior extension into iris root and lens, degree of circumferential spread (number of clock hours), posterior extension towards ciliary processes and peripheral retina, presence of cyclitic membranes and supraciliary effusion. It is now believed by many uveitis practitioners that UBM is mandatory in any case of presumed trematode induced uveitis in order to detect any subclinical CBG especially in cases associated with vitritis.
Other investigations that might be needed include ocular ultrasound in cases of media opacity to detect posterior segment complications and retinal imaging in the form of fundus fluorescein angiography (FFA) and macular optical coherence tomography (OCT) in cases of PTIGIU.
Unfortunately, due to the low yield of metagenomic sequencing on excised samples, the unavailability of the test in some countries and the high cost of the test, ocular sampling is no longer used to diagnose these cases except for research purposes.
Differential Diagnosis
There is a wide range of differential diagnosis with toxocariasis on the top of the list in cases of presumed trematode CBG. Other differential diagnosis include causes of granulomatous uveitis such as tuberculosis and toxoplasmosis. In young patients with "white" quiet eyes externally, masquerades should be excluded such as leukemic infiltrations.
Management
Add text here
General Treatment
Add text here
Medical Therapy
Add text here
Medical Follow-up
Add text here
Surgery
Add text here
Surgical Follow-up
Add text here
Complications
Add text here
Prognosis
Add text here
Additional Resources
Add text here
References
Add text here