Peripapillary Hyperreflective Ovoid Mass-Like Structures (PHOMS)
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Introduction
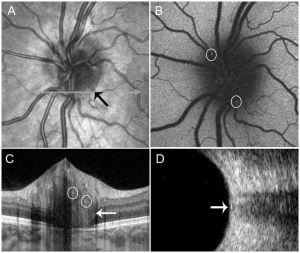
Peripapillary hyperreflective ovoid mass-like structures (PHOMS) are an enhance depth imaging optical coherence tomography (EDI-OCT) finding that may occur in isolation or in association with several disorders affecting the optic nerve. PHOMS are seen on EDI OCT as strictly peripapillary, mass-like structures that sit on top of Bruch’s membrane with upward deflection of overlying retinal layers that are hyperreflective throughout with a signal similar to the retinal nerve fiber layer.[1][2] PHOMS are thought to be nerve fiber bulging/herniation in the peripapillary region, assuming the shape of a "doughnut" or torus around the optic nerve head (ONH) and may indicate axoplasmic stasis.[3] PHOMS were previously considered a subtype buried optic disc drusen, but are now recognized as a separate entity[1], although they do frequently occur together.
Historical Background
Previous studies regarding the evaluation of morphologic characteristics of optic disc drusen (ODD), as visualized using Spectral-Domain Optical Coherence Tomography (SD-OCT), tried to categorize ODD into two types - visible and buried - according to their position related to the ONH. The visible ODD were primarily located inside the disc and were characterized by highly reflective borders with an internal hyporreflective core ("cysts") and RNFL thinning, often existing as multiple lumps, sometimes coalescing into larger aggregates. Buried optic nerve head drusen were located exclusively outside the ONH and were characterized by an amorphous C-shaped mass with relatively less distinct borders.[4]
Peripapillary Hyperreflective Ovoid Mass-Like Structures (PHOMS) are homogenous rounded structures, more recently described in studies regarding Optic Nerve Head (ONH) evaluation, that were initially considered a finding in Optic Disc Drusen (ODD). However, the Optic Disc Drusen Studies (ODDS) Consortium, a group of fellowship-trained neuro-ophthalmologists and researchers, elaborated on a recent work using Enhanced-Depth Imaging Optical Coherence Tomography (EDI-OCT) of ODD patients, in which they considered that PHOMS are not unique to ODD or papilledema and should not be used to differentiate these optic nerve head findings.[1]
Definition
ODDS Consortium Recommendations
Malmqvist et al. showed in 2018 that PHOMS were present in 74% of patients with optic disc drusen (ODD) included in their analysis. They stated that, despite their histopathological relationship not being clear, several features appeared to distinguish PHOMS from ODD:[1]
- Unlike ODD, PHOMS are hyperreflective without a sharp outer margin or hyporeflective core;
- PHOMS often lie external to and surrounding large parts of the disc, corresponding to funduscopically recognized pseudopapilledema;
- PHOMS do not demonstrate autofluorescence;
- PHOMS are not visible on B-scan ultrasound despite their superficial location;
- PHOMS can be seen in patients with papilledema without ODD;
- Histopathology of papilledema suggests that PHOMS might correspond to the lateral bulging or herniation of distended axons into the peripapillary retina.
Thus, PHOMS should not be mistaken for ODD.[1]
Multi-Rater Validation of PHOMS
The number of PHOMS' related papers has increased since the ODDS Consortium Recommendations. A Delphi consensus process to develop a consistent and refined definition of PHOMS focused on three characteristics found on OCT B-Scans:[2]
- Location: strictly peripapillary and sitting on top of Bruch’s membrane. Frequently, a gap can be observed in the B-scans of PHOMS aligned through the center of the optic disc;
- Effect on adjacent retinal layers: an upward deflection of at least two of the other retinal layers;
- Signal appearance: similar to the reflectivity of the retinal nerve fiber and ganglion cell layers.
Epidemiology
PHOMS were detected in 1.6% of adult participants in the population-based Beijing Eye Study and were most commonly associated with small optic discs.[5] Reports of the prevalence of PHOMS found in smaller adult control groups in other published studies range from to 0-18.9%.[6][7][8] The prevalence of PHOMS in a population-based Danish cohort of children without optic disc drusen or optic disc edema is 8.9%[9] and 26% in Danish children 6-12 years with >1.00 D of myopia.[10] In children with PHOMS, over half present with bilateral finding.[11][9] PHOMS are a particularly common cause of pseudopapilledema in children, with studies estimating that they account for between 53.6% and 98.4% of cases of pseudopapilledema in children.[12][11][13] PHOMS are common in children in otherwise normal discs (55%) as well as in eyes with other known causes of pseudopapilledema (81%) and in cases of true papilledema (67%).[11] Thus, the presence of PHOMS does not alone rule out optic disc edema. As PHOMS are not as easily detected on ultrasound and non-EDI OCT, they may be missed in children, leading to misdiagnosis.[12][14]
Etiology
PHOMS correspond to laterally bulging herniation of the optic nerve fibers and are thought to be a marker of axoplasmic stasis.[3][15] Histologic evaluation of the optic nerve head in patients with PHOMS have found swollen and vacuolated optic nerve fibers curving and expanding above the basement membrane opening.[15] Markers of stalled axoplasmic flow transport have been found within the distended axons on both radioisotopes and electron microscopy. [15] Differences in the pathologic manifestations of PHOMS indicate possible different mechanisms for their formation.[15] PHOMS occurring in association with ODD are hypothesized to be caused by axoplasmic stasis resulting from acellular and calcified deposits anterior to the lamina cribrosa.[16] Tilted disc syndrome leads to protrusion of Bruch membrane on the nasal side of the optic disc, resulting in limited axoplasmic flow due to bending of the nerve fibers.[3][17] Elevated intracranial pressure with resulting decreased axoplasmic flow is thought to cause PHOMS associated with papilledema.[18]
Impairment of glymphatic drainage or a translaminar pressure gradient have also been hypothesized as possible mechanisms in the development of PHOMS.[6]
Associated Conditions
As noted above, the PHOMS on OCT can be seen in individuals without any known ocular pathology as well as in multiple clinical situations such as:
- Papilledema,[19][20]
- Anterior Ischemic Optic Neuropathy (AION),[20]
- Central Retinal Vein Occlusion (CRVO),[20]
- Optic Neuritis (ON),[20]
- Tilted-Disc Syndrome (TDS),[20][21]
- Optic Disc Drusen (ODD),[1][12][4][20][22]
- Stickler Syndrome,[23]
- Nonarteritic Ischemic Optic Neuropathy (NAION),[8]
- Myopic Shift[21]
- Leber hereditary optic neuropathy (LHON)[15]
- Multiple Sclerosis[2][24]
- AQP4-IgG-positive neuromyelitis optica spectrum disease (NMOSD)[7]
- MOG-IgG-associated disease (MOGAD)[7]
Thus, PHOMS are a non-specific finding that can be related to true disc edema or cases of pseudopapilledema and usually resolves with its underlying condition/etiology.[3] Adult participants in the population-based Beijing Eye Study were not found to have major ocular or systemic diseases in association with PHOMS; however small/ crowded optic discs had a higher prevalence of PHOMS.[5] PHOMS associated with intracranial hypertension are noted to be larger in size. [25][26]
Diagnostic Modalities
EDI-OCT
PHOMS was first described as a finding on EDI-OCT images of patients with ODD. The resemblance of PHOMS with the histopathology sample of a patient with papilledema (lateral bulging/herniation of RNFL) presented the idea that both findings could be related to axoplasmic stasis.[1]
A retrospective cohort study that analyzed 64 eyes (32 patients, children aged 5-16 years) suspected of having pseudopapilledema (normal RNFL thickness, without disc swelling appearance on fundoscopy) found that 93.8% of eyes with PHOMS exclusively had small hyperreflective foci inside, indicating that it might contain calcium deposits in its composition. This finding supported the thesis that PHOMS and ODD might represent a spectrum of the same disease with PHOMS representing continued axoplasmic stasis leading to extrusion of calcified mitochondria, calcium deposition, and formation of ODD. They also identified a distinct ring sign visible on the infrared images of all cases of PHOMS, corresponding to the edge of the structure as seen in EDI-OCT.[13]
Features on EDI-OCT:
- C-shaped halo or torrus surrounding the optic disc[13] with PHOMS preferentially located nasally and superiorly.[9] PHOMS present as one contiguous structure.[9]
- Hyperreflective with internally slightly uneven hyperreflectivity. The reflectivity is similar to the RNFL. In children small internal hyperrflective spots are very common and thought to be from calcium deposition.[13]
- Controversial impact on overlying RNFL thinning.[15]
Infrared reflectance
Shows a ring structure that correlates with the blurred disc margin.[15]
OCTA
Complex vascular structures have been noted within PHOMS, possibly due to displacement of the deeper vessels in the optic disc into the retina or neovascularization. [8][27] Large PHOMS may lead to decrease in overlying vessel density within the optic nerve head.[12]
Autofluorescence
Hyperreflective spots noted inside PHOMS can be seen as hyperautofluorescent spots on FA.[13]
B-scan ultrasound
PHOMS in children have high echoes without posterior sound shadow at the retinal level of the optic disc that can be seen with a minimum gain of 56 dB unless large.[13]
Ocular Impacts and Prognosis
Vision
Adult participants in the population-based Beijing Eye Study were not found to have a decrease in visual function in association with PHOMS.[5]
Retinal Nerve Fiber Layer
Reports regarding the impact of PHOMS on RNFL thickness are varied. Adult participants in the population-based Beijing Eye Study were not found to have a decrease in retinal nerve fiber layer thickness nor optic nerve damage in association with PHOMS.[5] Pediatric patients with concurrent PHOMS and deep optic disc drusen have been found to have an increase in thickness of the RNFL which decreases as ODD become more superficial with age in some studies[28] and no effect on RNFL thickness in others.[6][22][29]
Natural History
In patients with PHOMS and IIH, PHOMS have been noted to decrease in size or even disappear in both children and adults when the elevated intracranial pressure is treated.[3][30] PHOMS of other underlying etiologies may also resolve along with resolution of their source.
Summary
PHOMS are an OCT finding that is nonspecific and can be seen in a number of optic nerve conditions including ODD and true disc edema. The presence of PHOMS should not be used to diagnose ODD. Clinicians should be aware of the significance and OCT appearance of PHOMS.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Malmqvist L, Bursztyn L, Costello F, Digre K, Fraser JA, Fraser C, Katz B, Lawlor M, Petzold A, Sibony P, Warner J, Wegener M, Wong S, Hamann S. The Optic Disc Drusen Studies Consortium Recommendations for Diagnosis of Optic Disc Drusen Using Optical Coherence Tomography. J Neuroophthalmol. 2018 Sep;38(3):299-307. doi: 10.1097/WNO.0000000000000585. PMID: 29095768.
- ↑ 2.0 2.1 2.2 Axel Petzold, Valerie Biousse, Lulu Bursztyn, Fiona Costello, Alison Crum, Kathleen Digre, Clare Fraser, J. Alex Fraser, Bradley Katz, Neringa Jurkute, Nancy Newman, Jette Lautrup-Battistini, Mitchell Lawlor, Petra Liskova, Birgit Lorenz, Lasse Malmqvist, Jason Peragallo, Patrick Sibony, Prem Subramanian, Robert Rejdak, Katarzyna Nowomiejska, Valerie Touitou, Judith Warner, Marianne Wegener, Sui Wong, Patrick Yu-Wai-Man & Steffen Hamann (2020) Multirater Validation of Peripapillary Hyperreflective Ovoid Mass-like Structures (PHOMS), Neuro-Ophthalmology, 44:6, 413-414, DOI: 10.1080/01658107.2020.1760891
- ↑ 3.0 3.1 3.2 3.3 3.4 Fraser JA, Sibony PA, Petzold A, Thaung C, Hamann S; ODDS Consortium. Peripapillary Hyper-reflective Ovoid Mass-like Structure (PHOMS): An Optical Coherence Tomography Marker of Axoplasmic Stasis in the Optic Nerve Head. J Neuroophthalmol. 2021 Dec 1;41(4):431-441. doi: 10.1097/WNO.0000000000001203. PMID: 33630781.
- ↑ 4.0 4.1 Lee KM, Woo SJ, Hwang JM. Morphologic characteristics of optic nerve head drusen on spectral-domain optical coherence tomography. Am J Ophthalmol. 2013 Jun;155(6):1139-1147.e1. doi: 10.1016/j.ajo.2013.01.024. Epub 2013 Mar 19. PMID: 23522355.
- ↑ 5.0 5.1 5.2 5.3 Jonas JB, Panda-Jonas S, Milea D, Lamirel C, Xu J, Jonas RA, Wang YX. Peripapillary Hyperreflective Ovoid Mass-Like Structures: Prevalence and Associations in the Adult Population of the Beijing Eye Study. Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):63. doi: 10.1167/iovs.66.6.63. PMID: 40540258; PMCID: PMC12184797.
- ↑ 6.0 6.1 6.2 Petzold A, Coric D, Balk LJ, Hamann S, Uitdehaag BMJ, Denniston AK, Keane PA, Crabb DP. Longitudinal Development of Peripapillary Hyper-Reflective Ovoid Masslike Structures Suggests a Novel Pathological Pathway in Multiple Sclerosis. Ann Neurol. 2020 Aug;88(2):309-319. doi: 10.1002/ana.25782. Epub 2020 Jun 9. PMID: 32426856; PMCID: PMC7496959.
- ↑ 7.0 7.1 7.2 Gernert JA, Wicklein R, Hemmer B, Kümpfel T, Knier B, Havla J. Peripapillary hyper-reflective ovoid mass-like structures (PHOMS) in AQP4-IgG-positive neuromyelitis optica spectrum disease (NMOSD) and MOG-IgG-associated disease (MOGAD). J Neurol. 2023 Feb;270(2):1135-1140. doi: 10.1007/s00415-022-11381-8. Epub 2022 Oct 16. PMID: 36245037; PMCID: PMC9886610.
- ↑ 8.0 8.1 8.2 Wang W, Liu J, Xiao D, Yi Z, Chen C. Features of Peripapillary Hyperreflective Ovoid Mass-Like Structures in Nonarteritic Anterior Ischemic Optic Neuropathy Patients and Normal Controls. Transl Vis Sci Technol. 2024;13(1):7. doi:10.1167/tvst.13.1.7
- ↑ 9.0 9.1 9.2 9.3 Behrens CM, Malmqvist L, Jørgensen M, Sibony PA, Munch IC, Skovgaard AM, Larsen M, Hamann S. Peripapillary Hyperreflective Ovoid Mass-like Structures (PHOMS) in Children: The Copenhagen Child Cohort 2000 Eye Study. Am J Ophthalmol. 2023 Jan;245:212-221. doi: 10.1016/j.ajo.2022.09.003. Epub 2022 Sep 13. PMID: 36108799.
- ↑ Hansen NC, Behrens CM, Hvid-Hansen A, Hamann S, Kessel L. Peripapillary hyperreflective ovoid mass-like structure (PHOMS): prevalence, risk factors, and development over time in Danish myopic children. J AAPOS. 2024 Dec;28(6):104034. doi: 10.1016/j.jaapos.2024.104034. Epub 2024 Nov 8. PMID: 39522589.
- ↑ 11.0 11.1 11.2 Pratt L, Rehan S, West J, Watts P. Prevalence of peripapillary hyperreflective ovoid mass-like structures (PHOMS) in suspected papilloedema in children. Eye (Lond). 2023 Oct;37(15):3209-3212. doi: 10.1038/s41433-023-02489-w. Epub 2023 Mar 11. PMID: 36906695; PMCID: PMC10564775.
- ↑ 12.0 12.1 12.2 12.3 Ahn YJ, Park YY, Shin SY. Peripapillary hyperreflective ovoid mass-like structures (PHOMS) in children. Eye (Lond). 2022 Mar;36(3):533-539. doi: 10.1038/s41433-021-01461-w. Epub 2021 Mar 17. PMID: 33731891; PMCID: PMC8873397.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Mezad-Koursh D, Klein A, Rosenblatt A, Teper Roth S, Neudorfer M, Loewenstein A, Iglicki M, Zur D. Peripapillary hyperreflective ovoid mass-like structures-a novel entity as frequent cause of pseudopapilloedema in children. Eye (Lond). 2021 Apr;35(4):1228-1234. doi: 10.1038/s41433-020-1067-x. Epub 2020 Jul 2. PMID: 32616868; PMCID: PMC8115042.
- ↑ Merchant KY, Su D, Park SC, Qayum S, Banik R, Liebmann JM, Ritch R. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology. 2013 Jul;120(7):1409-14. doi: 10.1016/j.ophtha.2012.12.035. Epub 2013 Mar 24. PMID: 23531353.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 Xiao D, Lhamo T, Meng Y, Xu Y, Chen C. Peripapillary hyperreflective ovoid mass-like structures: multimodal imaging and associated diseases. Front Neurol. 2024 Mar 28;15:1379801. doi: 10.3389/fneur.2024.1379801. PMID: 38606274; PMCID: PMC11006981.
- ↑ Skougaard M, Heegaard S, Malmqvist L, Hamann S. Prevalence and histopathological signatures of optic disc drusen based on microscopy of 1713 enucleated eyes. Acta Ophthalmol. 2020 Mar;98(2):195-200. doi: 10.1111/aos.14180. Epub 2019 Jul 1. PMID: 31264343.
- ↑ Pichi F, Romano S, Villani E, Lembo A, Gilardoni F, Morara M, Ciardella AP, Ohno-Matsui K, Nucci P. Spectral-domain optical coherence tomography findings in pediatric tilted disc syndrome. Graefes Arch Clin Exp Ophthalmol. 2014 Oct;252(10):1661-7. doi: 10.1007/s00417-014-2701-8. Epub 2014 Jul 20. PMID: 25038908.
- ↑ Hayreh SS. Pathogenesis of optic disc edema in raised intracranial pressure. Prog Retin Eye Res. 2016 Jan;50:108-44. doi: 10.1016/j.preteyeres.2015.10.001. PMID: 26453995; PMCID: PMC4698254.
- ↑ Wibroe EA, Malmqvist L, Hamann S. OCT Based Interpretation of the Optic Nerve Head Anatomy and Prevalence of Optic Disc Drusen in Patients with Idiopathic Intracranial Hypertension (IIH). Life (Basel). 2021 Jun 19;11(6):584. doi: 10.3390/life11060584. PMID: 34205357; PMCID: PMC8234108.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 Malmqvist L, Sibony PA, Fraser CL, Wegener M, Heegaard S, Skougaard M, Hamann S; Optic Disc Drusen Studies Consortium. Peripapillary Ovoid Hyperreflectivity in Optic Disc Edema and Pseudopapilledema. Ophthalmology. 2018 Oct;125(10):1662-1664. doi: 10.1016/j.ophtha.2018.04.036. Epub 2018 Jun 8. PMID: 29891127.
- ↑ 21.0 21.1 Lyu, I.J., Park, KA. & Oh, S.Y. Association between myopia and peripapillary hyperreflective ovoid mass-like structures in children. Sci Rep 10, 2238 (2020). https://doi.org/10.1038/s41598-020-58829-3
- ↑ 22.0 22.1 Teixeira FJ, Marques RE, Mano SS, Couceiro R, Pinto F. Optic disc drusen in children: morphologic features using EDI-OCT. Eye (Lond). 2020 Sep;34(9):1577-1584. doi: 10.1038/s41433-019-0694-6. Epub 2019 Nov 19. PMID: 31745329; PMCID: PMC7608464.
- ↑ Khatib TZ, Safi A, Nixon TRW, Georgoulas S, Montesano G, Martin H, Richards AJ, McNinch A, Poulson AV, Alexander P, Snead MP. Peripapillary Hyperreflective Ovoid Mass-Like Structures in Stickler Syndrome. Ophthalmol Retina. 2024;8(10):1013-1020. doi:10.1016/j.oret.2024.05.008
- ↑ Wicklein R, Wauschkuhn J, Giglhuber K, Kümpfel T, Hemmer B, Havla J, Knier B. Association of peripapillary hyper-reflective ovoid masslike structures and disease duration in primary progressive multiple sclerosis. Eur J Neurol. 2021 Dec;28(12). doi: 10.1111/ene.15056. Epub 2021 Aug 25. PMID: 34374178.
- ↑ Jørgensen M, Malmqvist L, Hansen AE, Fraser JA, Hamann S. Volumetric Measurement of Peripapillary Hyperreflective Ovoid Masslike Structures in Patients with Optic Disc Drusen. Ophthalmol Sci. 2021 Dec 21;2(1):100096. doi: 10.1016/j.xops.2021.100096. PMID: 36246173; PMCID: PMC9562331.
- ↑ Gernert JA, Christmann T, Kaufmann E, Delazer L, Kirsch I, Levin J, Schönecker S, Fietzek UM, Eulenburg PZ, Velten T, Gripshi M, Parhofer KG, Maier EM, Kümpfel T, Lotz-Havla AS, Havla J. Characterization of Peripapillary Hyperreflective Ovoid Mass-like Structures in a Broad Spectrum of Neurologic Disorders. Ophthalmology. 2025 May;132(5):590-597. doi: 10.1016/j.ophtha.2024.12.013. Epub 2024 Dec 14. PMID: 39675637.
- ↑ Borrelli E, Barboni P, Battista M, Sacconi R, Querques L, Cascavilla ML, Bandello F, Querques G. Peripapillary hyperreflective ovoid mass-like structures (PHOMS): OCTA may reveal new findings. Eye (Lond). 2021 Feb;35(2):528-531. doi: 10.1038/s41433-020-0890-4. Epub 2020 Apr 28. PMID: 32346103; PMCID: PMC7997888.
- ↑ Vienne-Jumeau A, Lebranchu P, Akhenak I, Bremond-Gignac D, Robert MP. Peripapillary hyperreflective ovoid mass-like structure (PHOMS) and optic disc drusen in pediatric pseudo-papilledema. Graefes Arch Clin Exp Ophthalmol. 2025 Jun;263(6):1725-1732. doi: 10.1007/s00417-025-06799-5. Epub 2025 Mar 18. PMID: 40102220.
- ↑ Wicklein R, Wauschkuhn J, Giglhuber K, Kümpfel T, Hemmer B, Havla J, Knier B. Association of peripapillary hyper-reflective ovoid masslike structures and disease duration in primary progressive multiple sclerosis. Eur J Neurol. 2021 Dec;28(12). doi: 10.1111/ene.15056. Epub 2021 Aug 25. PMID: 34374178.
- ↑ Chang YH, Gise R, Estrela T, Zurakowski D, Staffa SJ, Jeon-Chapman J, Dagi LR. Peripapillary hyperreflective ovoid mass-like structures before and after intracranial decompression: a longitudinal cohort study. Ophthalmology. 2025 Jul 28:S0161-6420(25)00459-2. doi: 10.1016/j.ophtha.2025.07.025. Epub ahead of print. PMID: 40738333.