Choroidal Neovascularization: OCT Angiography Findings
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Disease
Choroidal neovascularization (CNV) is part of the spectrum of exudative age-related macular degeneration (AMD) that consists of an abnormal growth of vessels from the choroidal vasculature to the neurosensory retina through the Bruch's membrane.[1] CNV can also develop in a number of other conditions such as myopic degeneration, chronic central serous chorioretinopathy, macular telangiectasia type 2, various white dot syndromes and other uveitic processes, and some choroidal tumors.[2][3] Leakage of retinal edema and hemorrhage from CNV threatens visual acuity.
Etiology
Etiology of CNV is multifactorial. Alterations in Bruch's membrane, migration of macrophages and production of vascular endothelium growth factor (VEGF), play an important role in the development of this disease.[4][5][6][7]
Risk Factors
The incidence and progression of AMD are related to age and genetic factors.[8] With aging, the lysosomal activity for the degradation of external segments of photoreceptors decreases. This leads to subsequent accumulation of lipofuscin, which affects the normal function of the RPE. Another important risk factor for the development of CNV is the presence of large, confluent soft drusen.[9]
Oxidative stress may play an important role in AMD.[10] Several modifiable risk factors have been identified, including quitting smoking, dietary intake of omega-3 fatty acids, consuming vegetables and fruit with antioxidants including lutein and zeaxanthin, exercise, wearing sunglasses, and maintaining a healthy weight.[11]
Pathophysiology
Alterations in the normal transport of metabolites, ions and water through Bruch's membrane in AMD, alter the nutrition and stability of retinal pigment epithelium (RPE) from choriocapillaris and the transport of waste out from the neurosensory retina. Hypoxia leads to VEGF being released by the RPE, which initiates a cascade of angiogenic responses at the level of the choroidal endothelium. Bruch´s membrane damage is required to allow the passage of abnormal neovascular vessels from the choroidal vasculature through the breaks in Bruch’s membrane to the retina. This impairment is part of the pathological course of AMD.[12][13]
Classification
Histologically, neovascular membranes are classified into:
- Type 1 ("occult"), when the neovascular membrane is located below the RPE. Type 1 CNV demonstrates occult leakage on fluorescein angiography. Polypoidal choroidal vasculoplathy (PCV) is a subtype of Type 1 CNV that is characterized by the presence of polyp-like aneurysmal dilations of the branching vascular network.
- Type 2 ("classic"), passes through the RPE and is located above the RPE in the subretinal space. This is related to the angiographic classification of a classic CNV.
- Type 3 is defined as Retinal Angiomatous Proliferation (RAP), which corresponds to neovascularization that develops within the neurosensory retina an progresses posteriorly into the subretinal space.
Diagnosis
Clinical findings
In the presence of CNV, the patient experiences an acute decrease in visual acuity, relative scotoma, and/or metamorphopsia. The retinal examination shows a grayish macular lesion associated with subretinal fluid, macular edema, exudation, and/or hemorrhages.
Diagnostic Procedures
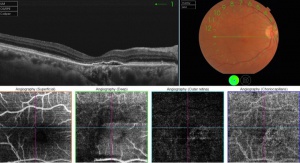
OCT Angiography
En face OCT angiography (OCTA) is a new technology that has the great ability to show the retinal and choroidal microcirculation in detail without invasive means.[14] Instead of intraocular contrast, it uses motion contrast by comparing the decorrelation signal between repeated B-scans obtained at a given retinal cross-section to detect blood flow. It utilizes the principle that theoretically only the circulating RBCs within the retinal vasculature would be moving between the B-scans. Each en face OCT angiogram is cross-registered with the corresponding OCT B-scans, an OCT thickness map, and a structural en face OCT, which allows for concurrent visualization of structure and blood flow. OCTA is available on both spectral domain and swept source OCT devices.[15]
The conventional CNV evaluation often consists of multimodal imaging with fluorescein angiography (FA) combined with indocyanine green angiography (ICGA) and OCT. These well-established modalities can guide effective CNV management but have their limitations, especially when characterizing vascular structures at different depths. For example, FA only allows for evaluation of the superficial capillary plexus within the retina. OCTA, on the other hand, allows for noninvasive three-dimensional analysis of the retinal and choroidal vasculature and can be segmented to view each of the vascular plexuses individually.[16]
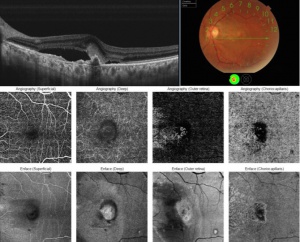
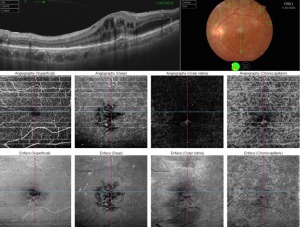
This ability to visualize vascular structures at different depths/layers of the retina makes OCTA a tool well suited to evaluate CNV. For the purpose of visualizing changes in eyes with CNV or suspected CNV, a segmentation of the outer retina (extending from the outer plexiform layer to Bruch’s membrane) and a segmentation of the choriocapillaris (approximately 20um thick region just below the RPE) are most useful. CNV can be seen as a seafan or coraliform neovascular complex within the outer retina, which is ordinarily devoid of blood flow in normal eyes. Type 1 CNV is observed in OCTA as a neovascular complex between the RPE and Bruch's membrane, originating in the choroid.[17] The type 2 CNV is visualized as a neovascular network that grows from the choroid vasculature and traverses the RPE-Bruch's membrane complex into the subretinal space.[18] Type 3 CNV is clinically seen as tiny intra- and subretinal hemorrhages that correlate on OCTA as an intraretinal anastomosis originating in the deep capillary plexus of the retina.
The PCV subtype of CNV can also be seen as a branching neovascular network within the outer retina but with concurrent aneurysmal dilations. After repeat pharmacologic intervention the large branches of the CNV become pruned and the smaller capillaries and any polyps may no longer be visualized (whether due to slow or absent flow, or complete regression). The choriocapillaris layer may demonstrate decreased flow or flow voids adjacent to the CNV complex. Additionally, choriocapillaris hypoperfusion may be seen underlying any areas of RPE atrophy.[19]
The use of OCTA in CNV imagining is not without challenges.[20] OCTA is susceptible to several imaging artifacts. Some of these, such as motion or signal attenuation, may be mitigated through patient instruction and technician training. Projection artifacts arise when blood flow of superficial layers of the retina is projected onto deeper structures below, which can cause false flow signal in deeper anatomical layers. CNV assessment suffers since proper visualization requires high-quality images of the outer retina, where projection artifacts are especially prominent due to proximity to the highly reflective RPE layer. Furthermore, the eruption of CNV can lead to segmentation artifact via the distortion of the retinal layers. This may require manual adjustment of the segmentation slabs, which may be time consuming and lower the repeatability of the test. Tools for automatic segmentation through deep learning and modality of OCTA such as projection-resolved OCTA (PR-OCTA) algorithm have been reported to improve the quality and repeatability of OCTA studies.[21][22]
Management
Treatment
Taking into account the numerous recent studies on the treatment of CNV in AMD, it has been shown that antiangiogenic therapy shows the best result both histologically with the regression of the neovascular lesion and functionally with improvement of the visual acuity. Although the treatment is the same for all types of CNV, it is important to differentiate them, since they do not all respond identically and some of them have a higher rate of recurrence.
References
- ↑ Turbert, D. (2020, March 18). What are Choroidal Neovascular membranes? Reviewed by Ninel Z Gregori, MD. American Academy of Ophthalmology. https://www.aao.org/eye-health/diseases/choroidal-neovascular-membranes.
- ↑ Cheung C. et al. “Myopic Choroidal Neovascularization: Review, Guidance, and Consensus Statement on Management”. Ophthalmology. 2017 Nov;124(11):1690-1711. doi: 10.1016/j.ophtha.2017.04.028
- ↑ Agarwal A. et al. “An update on inflammatory choroidal neovascularization: epidemiology, multimodal imaging, and management”. J Ophthalmic Inflamm Infect. Vol: 8(13). doi: 10.1186/s12348-018-0155-6
- ↑ Kennedy CJ, Rakoczy PE, Constable IJ. Lipofuscin of the retinal pigment epithelium: a review. Eye 1995;9:763–71.
- ↑ Rakoczy PE, Zhang D, Robertson T, et al. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am J Pathol 2002;161:1515–24
- ↑ Hageman GS, Mullins RF. Molecular composition of drusen as related to substructural phenotype. Mol Vis 1999;5:28.
- ↑ Spillsbury K. et al. “Overexpression of Vascular Endothelial Growth Factor (VEGF) in the Retinal Pigment Epithelium Leads to the Development of Choroidal Neovascularization”. Am J Pathol. 2000 Jul; 157(1): 135–144. doi: 10.1016/S0002-9440(10)64525-7
- ↑ Klein R, Klein BE, Jensen SC, et al. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study [see comments]. Ophthalmology 1997;104:7–21.
- ↑ Mullins RF, Russell SR, Anderson DH, et al. Drusen associated with aging and age-related macular degeneration contain proteins common to extracel- lular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J 2000;14:835–46.
- ↑ Sarks JP, Sarks SH, Killingsworth MC. Evolution of soft drusen in age-related macular degeneration. Eye 1994;8:269–83.
- ↑ The Age-Related Eye Disease Study 2 (AREDS2) Research Group*. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA. 2013;309(19):2005–2015. doi:10.1001/jama.2013.4997
- ↑ Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation- induced regression. Surv Ophthalmol 1999;44:1–29.
- ↑ Tombran-Tink J, Shivaram SM, Chader GJ, et al. Expression, secretion, and age-related downregulation of pigment epithelium-derived factor, a serpin with neurotrophic activity. J Neurosci 1995;15:4992–5003.
- ↑ de Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122(6):1228-38.
- ↑ Spaide RF. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am J Ophthalmol. 2015;160(1):6–16.
- ↑ Carnevali A, Cicinelli MV, Capuano V, Corvi F, Mazzaferro A, Querques L, et al. Optical Coherence Tomography Angiography: A Useful Tool for Diagnosis of Treatment-Naïve Quiescent Choroidal Neovascularization. Am J Ophthalmol [Internet]. 2016;169:189–98. Available from: http://dx.doi.org/10.1016/j.ajo.2016.06.042
- ↑ Kuehlewein, L, Bansal, M, Lenis, TL. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. Am J Ophthalmol 2015;160(4):739–48.e2.
- ↑ Lumbroso B, Rispoli M, Savastano MC. Longitudinal Optical Coherence Tomography–Angiography Study of Type 2 Naive Choroidal Neovascularization Early Response After Treatment. Retina [Internet]. 2015;35(11):2242–51.
- ↑ de Carlo TE, Kokame GT, Shantha JG, Lai JC, Wee R. Spectral-Domain Optical Coherence Tomography Angiography for the Diagnosis and Evaluation of Polypoidal Choroidal Vasculopathy. Ophthalmologica. 2018;239(2-3):103-9.
- ↑ Holmen IC, Konda SM, Pak JW, et al. Prevalence and Severity of Artifacts in Optical Coherence Tomographic Angiograms. JAMA Ophthalmol. 2020;138(2):119–126. doi:10.1001/jamaophthalmol.2019.4971
- ↑ Jie Wang, Tristan T. Hormel, Liqin Gao, Pengxiao Zang, Yukun Guo, Xiaogang Wang, Steven T. Bailey, and Yali Jia, "Automated diagnosis and segmentation of choroidal neovascularization in OCT angiography using deep learning," Biomed. Opt. Express 11, 927-944 (2020)
- ↑ Hormel TT, Huang D, Jia Y. Artifacts and artifact removal in optical coherence tomographic angiography. Quant Imaging Med Surg. 2021 Mar;11(3):1120-1133. doi: 10.21037/qims-20-730. PMID: 33654681; PMCID: PMC7829161.